Harnessing the Potential of JCARH125: Advancing BCMA-Targeted CAR T-Cell Therapy for Multiple Myeloma
Clinical trials involving BCMA-targeted CAR T cells have shown positive outcomes without off-target effects. Juno Therapeutics is advancing a CAR T-cell therapy, which includes both CD4+ and CD8+ T-cells from the same patient, modified to express a BCMA-specific CAR. This therapy utilizes a BCMA-specific single chain variable fragment (scFv) with human variable regions, a CD137 [4 1BB] co-stimulatory domain, and a CD3ζ signaling domain. The binding domain has been shown to bind BCMA specifically and with high affinity, without staining off-target cells.
When the CAR was expressed in primary T cells, they became activated, multiplied, and displayed potent lytic activity against BCMA-positive targets, while BCMA-negative targets were unaffected. The specific combination of the CAR's components was found to be advantageous. The CAR-transduced primary cells exhibited robust lytic activity and produced high levels of effector cytokines when in contact with target cells.
The CAR-expressing primary T cells demonstrated similar activity against both BCMA+ cell lines and primary CD138+ myeloma cells. They also showed comparable activity in the presence or absence of soluble BCMA. T cells engineered with the CAR from MM patients showed similar characteristics and antigen-specific activities as those from healthy donors.
In vitro assays showed no tonic signaling without antigen, but efficient signaling through the CAR was observed with increasing antigen levels. In vivo testing in NSG mouse models with MM cells showed enhanced survival and reduced tumor growth in mice treated with anti-BCMA CAR+ cells. In one model, complete tumor regression was observed by day 20 post CAR T-cell transfer, lasting up to day 60.
The circulating BCMA CAR T cells peaked on day 14 post transfer, with more CD8+ CAR+ T cells observed than CD4+ CAR+ T cells. These findings support the development of a fully human BCMA-specific CAR T cell product that targets myeloma cells across a range of antigen densities, unaffected by soluble BCMA levels. Phase 1 clinical trials for JCARH125, a BCMA CAR T cell candidate, are anticipated for early 2018.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
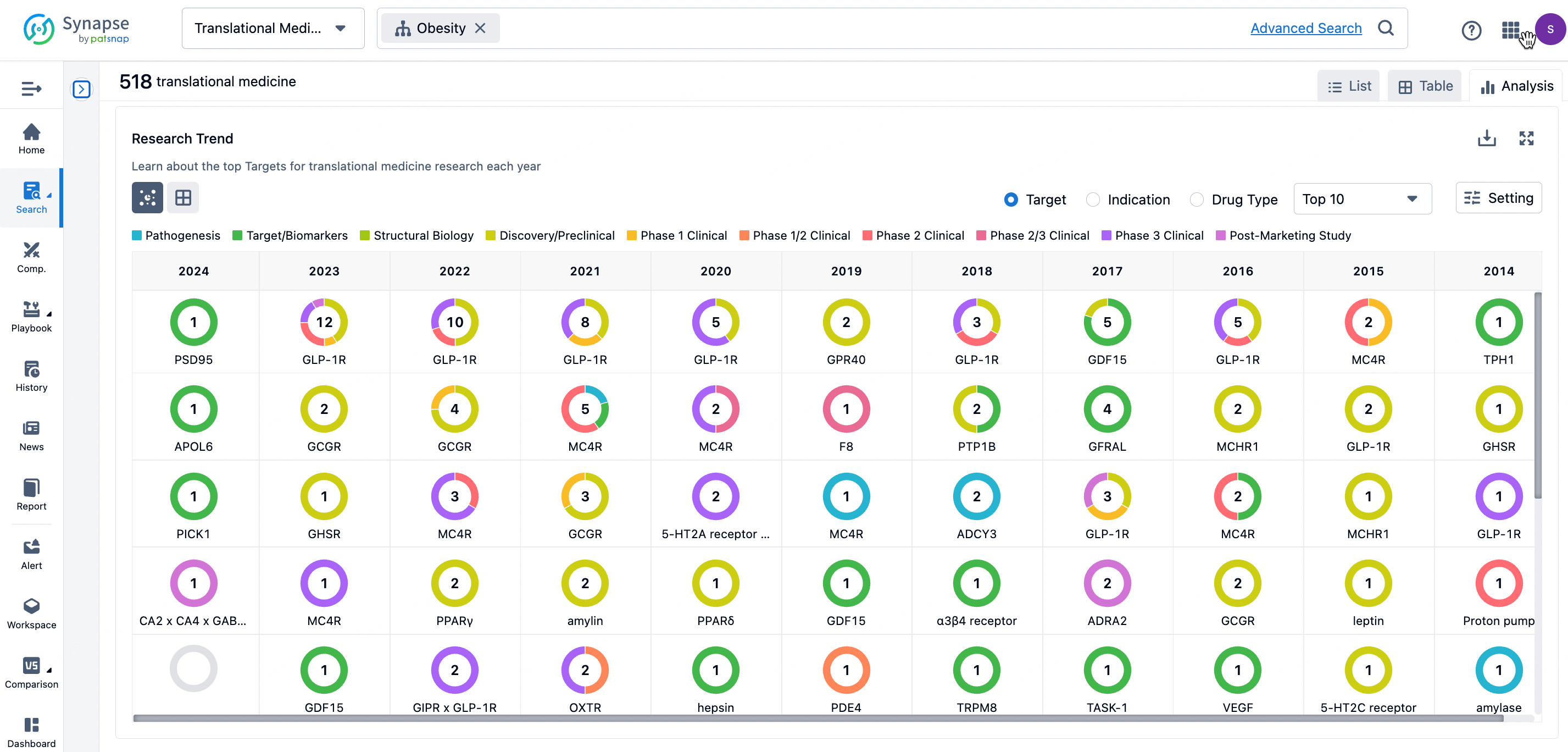
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.