A Comprehensive Review of Febuxostat's R&D Innovations
Febuxostat's R&D Progress
Febuxostat is a small molecule drug that primarily targets the enzyme xanthine oxidase (XO). It has been approved for use in various therapeutic areas, including immune system diseases, congenital disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, skin and musculoskeletal diseases, and other diseases. The drug is indicated for the treatment of arthritis, specifically gouty arthritis, as well as chronic hyperuricemia, tumor lysis syndrome, hyperuricemia, and gout.
Febuxostat was developed by Teijin Pharma Ltd., a pharmaceutical company based in Liechtenstein. It received its first approval in 2008 in Liechtenstein, making it available for use in that country. The drug has also obtained approvals in other countries, indicating its global reach and acceptance.
As a small molecule drug, Febuxostat is designed to inhibit the activity of XO, an enzyme involved in the production of uric acid. By reducing the levels of uric acid in the body, the drug aims to alleviate the symptoms associated with gout and other related conditions. Gout is a form of arthritis characterized by the buildup of uric acid crystals in the joints, leading to inflammation and severe pain.
The approval of Febuxostat in multiple therapeutic areas highlights its potential to address various medical conditions related to immune system disorders, metabolic diseases, and musculoskeletal disorders. Its effectiveness in treating chronic hyperuricemia and gouty arthritis positions it as a valuable option for patients suffering from these conditions. Febuxostat has reached the highest phase of development which is approved globally. This status further supports its credibility and potential for widespread use in the pharmaceutical market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Febuxostat: XO inhibitors
XO inhibitors are a class of drugs that inhibit the activity of the enzyme xanthine oxidase (XO). Xanthine oxidase is involved in the metabolism of purines, which are substances found in certain foods and also produced by the body. When purines are broken down, they form uric acid. However, excessive production of uric acid or impaired excretion can lead to elevated levels of uric acid in the blood, a condition known as hyperuricemia.
XO inhibitors work by blocking the action of xanthine oxidase, thereby reducing the production of uric acid. By lowering uric acid levels, XO inhibitors are used in the treatment of conditions such as gout, a type of arthritis caused by the deposition of uric acid crystals in the joints.
These inhibitors can help prevent the formation of uric acid crystals and alleviate the symptoms of gout, such as pain, swelling, and inflammation. They can also be used in the management of conditions associated with high uric acid levels, such as kidney stones and certain types of cancer.
It's important to note that XO inhibitors should be used under medical supervision, as they may have potential side effects and interactions with other medications. The specific XO inhibitor prescribed may vary depending on the individual's condition and medical history.
Drug Target R&D Trends for Febuxostat
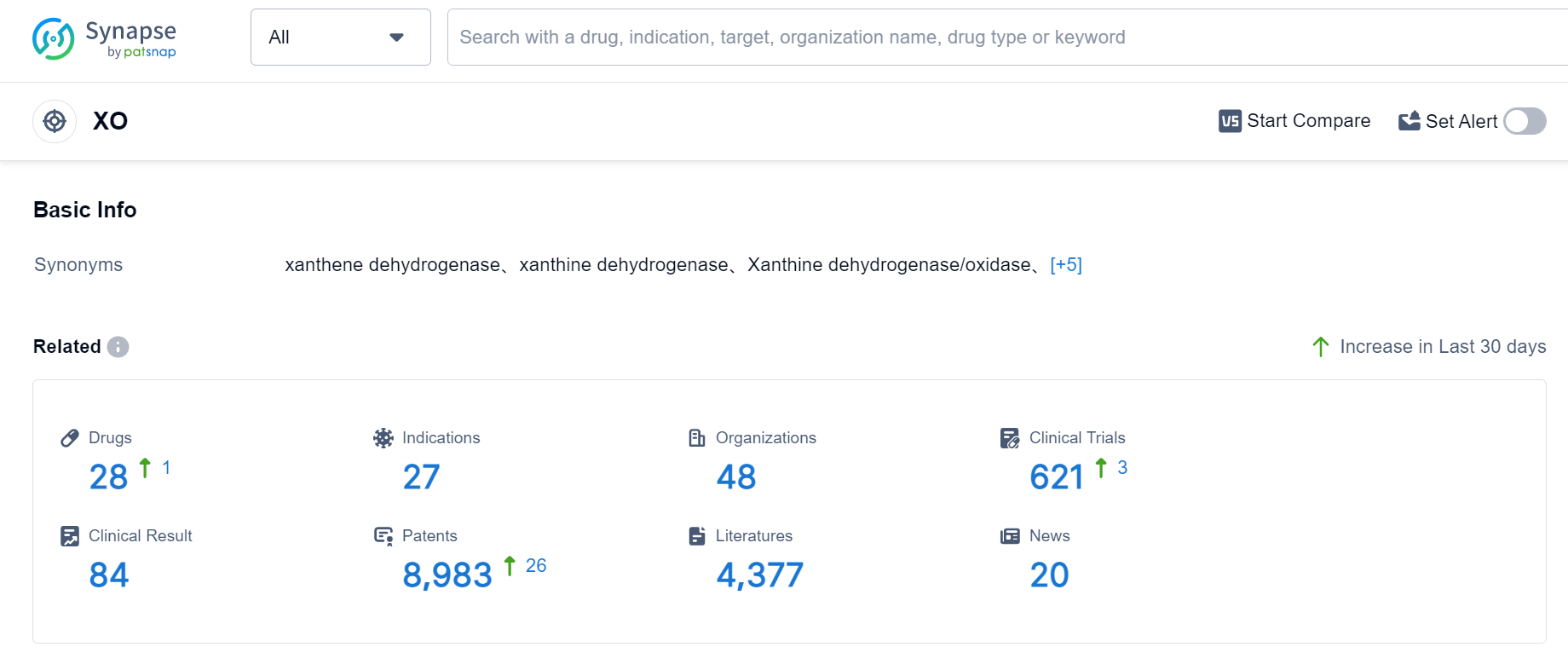
According to Patsnap Synapse, as of 5 Sep 2023, there are a total of 28 XO drugs worldwide, from 48 organizations, covering 27 indications, and conducting 621 clinical trials.
The current competitive landscape for target XO is characterized by multiple companies with drugs in various stages of development. The highest stage of development is the approved phase, indicating successful research and development efforts by several companies. The indications with the most approved drugs are Hyperuricemia and Gout, suggesting a focus on addressing these medical needs. Small molecule drugs are progressing rapidly, indicating their significance in the development of treatments for target XO. The presence of biosimilars, represented by unknown drug types, suggests intense competition around the innovative drugs. The United States, China, and Japan are leading in terms of drug development, with China showing notable progress. Overall, the target XO presents opportunities for further research and development, particularly in indications with fewer approved drugs and in countries/locations with emerging progress.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Febuxostat is a small molecule drug developed by Teijin Pharma Ltd. that targets the enzyme XO. It has been approved for use in various therapeutic areas and is indicated for the treatment of arthritis, chronic hyperuricemia, tumor lysis syndrome, hyperuricemia, and gout. Its first approval was obtained in Liechtenstein in 2008, and it has since gained global recognition. Febuxostat's approval status and its ability to address multiple medical conditions make it a promising drug in the field of biomedicine.