A Comprehensive Review of Hydrocortisone Sodium Succinate's R&D Innovations and Drug Target Mechanism
Hydrocortisone Sodium Succinate's R&D Progress
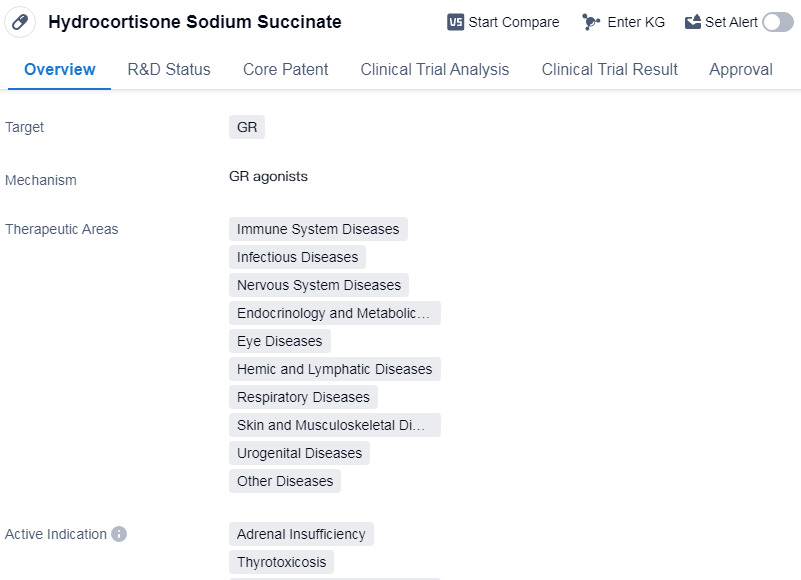
Hydrocortisone Sodium Succinate is a small molecule drug that primarily targets the glucocorticoid receptor (GR). It has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, nervous system diseases, endocrinology and metabolic diseases, eye diseases, hemic and lymphatic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
The drug is indicated for the treatment of adrenal insufficiency, thyrotoxicosis, eye diseases, hematologic diseases, hypersensitivity, etc.
Hydrocortisone Sodium Succinate was developed by Pfizer Japan, Inc. and has received approval in multiple countries. Its highest phase of development is approved, indicating that it has successfully completed clinical trials and demonstrated safety and efficacy. The drug was first approved in the United States in April 1955.
As a small molecule drug targeting the GR, Hydrocortisone Sodium Succinate is likely to exert its therapeutic effects by modulating the activity of the glucocorticoid receptor. This receptor is involved in various physiological processes, including immune response regulation, metabolism, and stress response. By targeting the GR, the drug may help regulate these processes and alleviate symptoms associated with the indicated diseases.
Given its broad range of therapeutic areas and indications, Hydrocortisone Sodium Succinate may have a significant impact on patients suffering from various diseases. Its long history of approval and use since 1955 suggests that it has been well-established and widely accepted in the medical community.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Hydrocortisone Sodium Succinate: GR agonists
From a biomedical perspective, GR agonists refer to substances or drugs that activate the glucocorticoid receptor (GR). The GR is a type of nuclear receptor found in cells that binds to the hormone cortisol and regulates various physiological processes, including metabolism, immune response, and stress response. GR agonists mimic the action of cortisol by binding to the receptor and triggering a cascade of cellular events. These agonists can be used therapeutically in conditions where the activation of GR is desired, such as in the treatment of inflammatory diseases, autoimmune disorders, and certain types of cancer. By activating the GR, these agonists can help modulate immune responses, reduce inflammation, and regulate gene expression.
Drug Target R&D Trends for Hydrocortisone Sodium Succinate
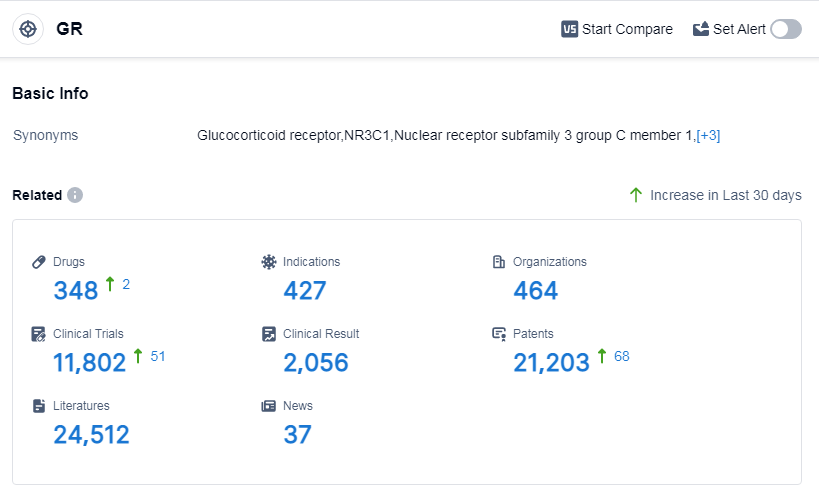
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 348 GR drugs worldwide, from 464 organizations, covering 427 indications, and conducting 11802 clinical trials.
The analysis of the target GR reveals a competitive landscape with several companies showing growth and development in this area. Novartis AG, Bausch Health Cos., Inc., and Shionogi & Co., Ltd. are among the top companies with a diverse pipeline and approved drugs. Eczema, asthma, psoriasis, and skin diseases are the indications with the highest number of approved drugs, indicating a focus on these areas. Small molecule drugs dominate the drug types, suggesting intense competition and innovation. The United States and China are the leading countries/locations in terms of development, with significant progress in both regions. China, in particular, has emerged as a strong player in the target GR. Overall, the target GR presents opportunities for further growth and development, with potential for breakthrough therapies in various indications and drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Hydrocortisone Sodium Succinate is a small molecule drug developed by Pfizer Japan, Inc. It targets the glucocorticoid receptor and has been approved for use in multiple therapeutic areas and indications. Its approval history and broad range of indications indicate its potential significance in the treatment of various diseases.