A Comprehensive Review of Lymecycline's R&D Innovations and Drug Target Mechanism
Lymecycline's R&D Progress
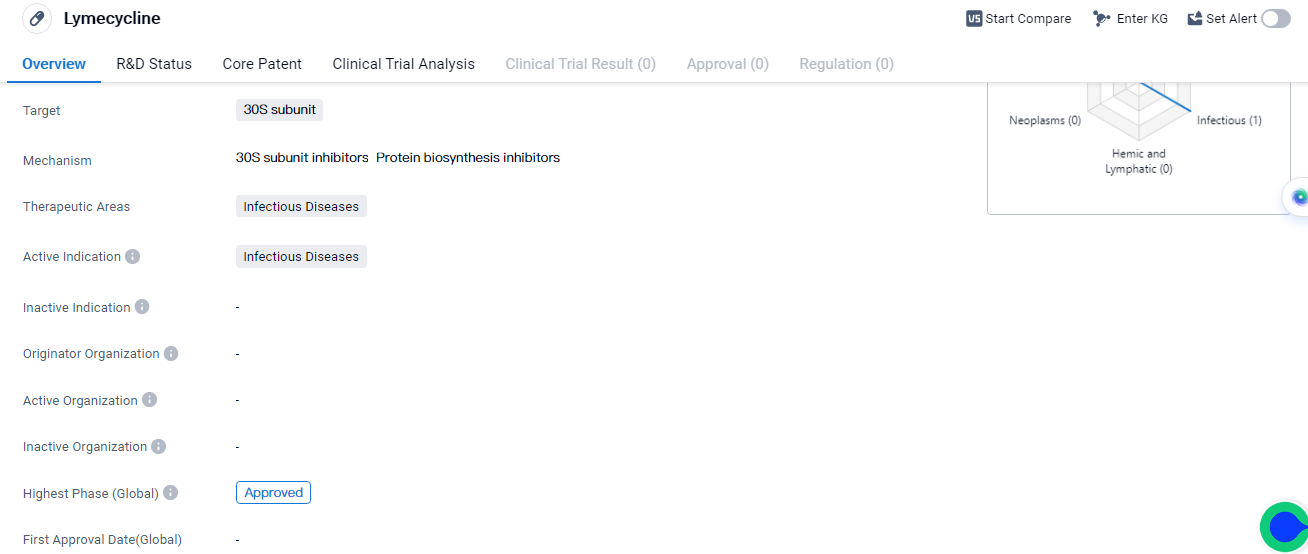
Lymecycline is a small molecule drug that falls under the category of biomedicine. It specifically targets the 30S subunit, which is a component of the ribosome involved in protein synthesis.
Lymecycline is specifically indicated for the treatment of infectious diseases. This suggests that it has been developed to combat a wide range of infections caused by bacteria or other pathogens. The drug's approval for use in the treatment of infectious diseases indicates that it has successfully undergone rigorous testing and evaluation, meeting the necessary safety and efficacy standards.
As a small molecule drug, Lymecycline is likely to have a relatively small molecular size, allowing it to easily penetrate cell membranes and to reach its target within the 30S subunit. This characteristic is advantageous as it enhances the drug's ability to effectively inhibit protein synthesis and halt the growth of infectious agents.
The fact that Lymecycline is in the highest phase of development, which is the approved phase, suggests that it has already undergone extensive clinical trials and regulatory review, and demonstrated its safety and efficacy profile, leading to its approval for use in the treatment of infectious diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lymecycline: 30S subunit inhibitors and protein biosynthesis inhibitors
30S subunit inhibitors are a type of drug that specifically target the 30S subunit of the bacterial ribosome, which is involved in protein synthesis. These inhibitors interfere with the function of the 30S subunit, preventing the synthesis of new proteins. By inhibiting protein biosynthesis, these drugs can effectively inhibit bacterial growth and replication. This class of drugs is commonly used in the treatment of bacterial infections and can be effective against a wide range of bacterial pathogens. Examples of 30S subunit inhibitors include aminoglycosides and tetracyclines.
Drug Target R&D Trends for Lymecycline
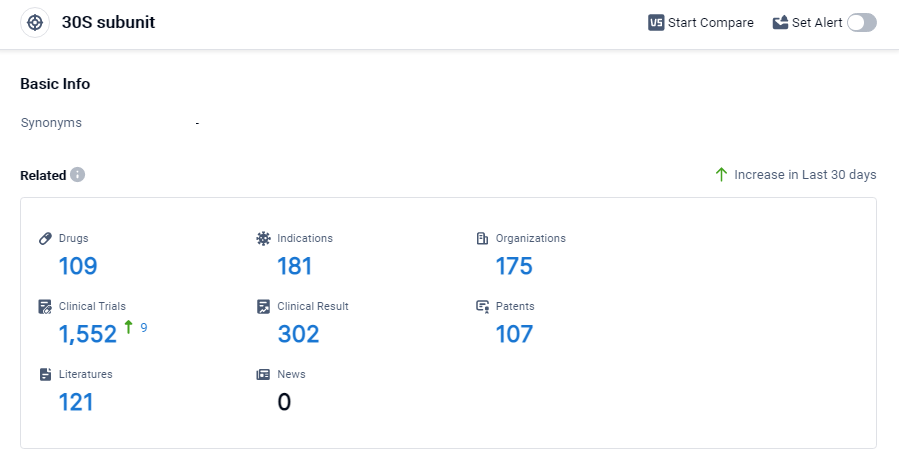
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 109 30S subunit drugs worldwide, from 175 organizations, covering 181 indications, and conducting 1552 clinical trials.
The analysis of the target 30S subunit reveals a competitive landscape with multiple companies focusing on research and development. Pfizer Inc. has the highest stage of development with 6 approved drugs. Bacterial infections are the most targeted indication, with a significant number of approved drugs. Small molecule drugs are progressing rapidly, indicating intense competition in this area. China is leading in terms of the number of approved drugs, followed by the United States and Japan. The future development of the target 30S subunit is promising, with ongoing research and development efforts focused on addressing various indications and drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Lymecycline is a small molecule drug that targets the 30S subunit and is primarily used in the treatment of infectious diseases. Its approval for use in therapeutic area indicates that it has successfully met the necessary safety and efficacy standards. As a small molecule drug, Lymecycline is likely to possess characteristics that enhance its ability to effectively inhibit protein synthesis and combat infectious agents.