A Comprehensive Review of Pralidoxime Chloride's R&D Innovations
Pralidoxime Chloride's R&D Progress
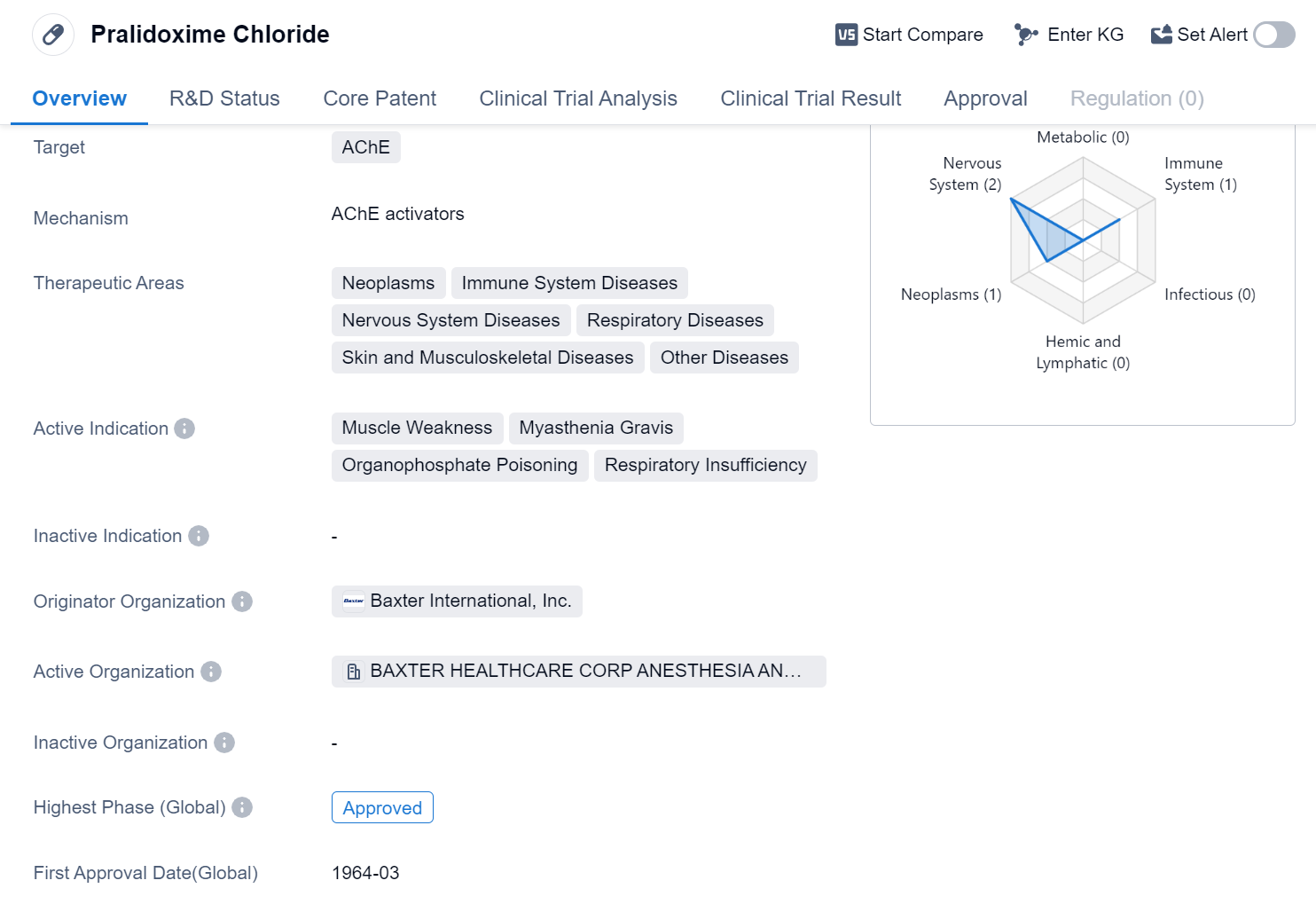
Pralidoxime Chloride is a small molecule drug that primarily targets Acetylcholinesterase (AChE). It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, nervous system diseases, respiratory diseases, skin and musculoskeletal diseases, and other diseases. The drug is indicated for the treatment of muscle weakness, myasthenia gravis, organophosphate poisoning, and respiratory insufficiency.
The originator organization of Pralidoxime Chloride is Baxter International, Inc. This pharmaceutical company played a crucial role in the development and approval of the drug. Pralidoxime Chloride has achieved the highest phase of approval globally, indicating its widespread acceptance and recognition in the medical community.
The drug received its first approval in the United States in March 1964, making it a well-established and long-standing treatment option. The approval in the United States also signifies the drug's compliance with the rigorous regulatory standards set by the country's regulatory authorities.
Pralidoxime Chloride's therapeutic applications cover a broad range of diseases. Its efficacy in treating muscle weakness, myasthenia gravis, organophosphate poisoning, and respiratory insufficiency highlights its importance in critical care and emergency medicine.
As a small molecule drug, Pralidoxime Chloride likely possesses favorable pharmacokinetic properties, allowing for efficient absorption, distribution, metabolism, and excretion within the body.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pralidoxime Chloride: AChE activators
AChE activators are substances that stimulate the activity of acetylcholinesterase (AChE), an enzyme responsible for breaking down the neurotransmitter acetylcholine in the body. From a biomedical perspective, AChE activators are often used in the treatment of conditions such as Alzheimer's disease and myasthenia gravis, where there is a deficiency or dysfunction of acetylcholine. By enhancing the activity of AChE, these activators help increase the breakdown of acetylcholine, leading to improved neurotransmission and symptom relief. It is important to note that AChE activators should be used under medical supervision due to their potential side effects and interactions with other medications.
Drug Target R&D Trends for Pralidoxime Chloride
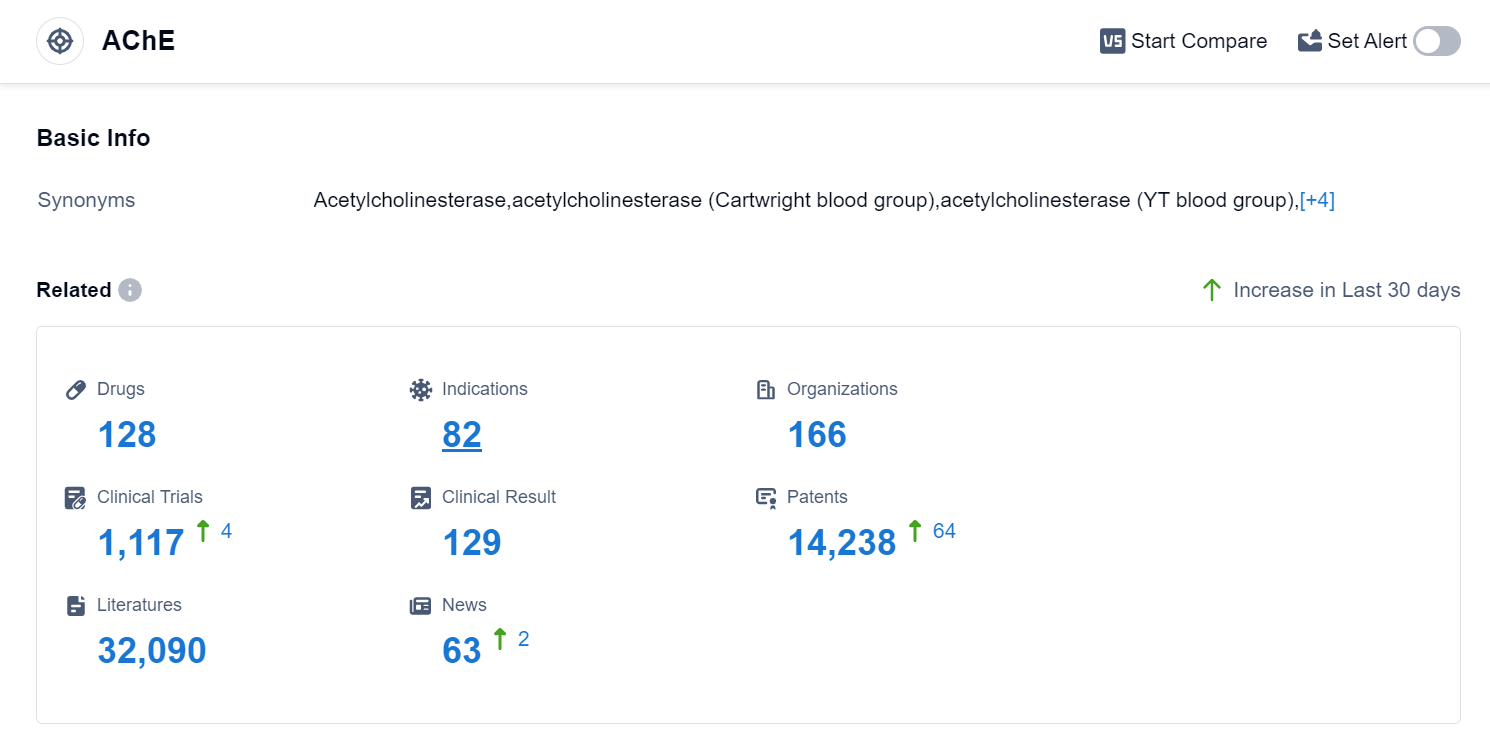
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 128 AChE drugs worldwide, from 166 organizations, covering 82 indications, and conducting 1117 clinical trials.
The analysis of the target AChE reveals a competitive landscape with multiple companies actively involved in R&D. AbbVie, Inc., Shanghai Pharmaceuticals Holding Co., Ltd., Pfizer Inc., Zeria Pharmaceutical Co., Ltd., and Eisai Co., Ltd. are among the companies growing fastest in this area. The approved drugs for the target AChE cover indications such as Myasthenia Gravis, Alzheimer Disease, Poisoning, Glaucoma, and more. Small molecule drugs dominate the drug types progressing rapidly under the target AChE, indicating intense competition. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target AChE, with China showing significant progress. Overall, the target AChE presents a promising area for pharmaceutical development, with potential applications in various neurological and gastrointestinal disorders.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pralidoxime Chloride is a small molecule drug developed by Baxter International, Inc. It targets AChE and has been approved for use in multiple therapeutic areas. Its active indications include muscle weakness, myasthenia gravis, organophosphate poisoning, and respiratory insufficiency. With its long history of approval and broad therapeutic applications, Pralidoxime Chloride is a valuable asset in the pharmaceutical industry, particularly in the treatment of various diseases affecting different body systems.