A2 Bio Reports Initial Patient Treatment in EVEREST-2 Phase 1 Trial for New Mesothelin Logic-Gated CAR T
A2 Biotherapeutics, Inc., a clinical-stage cell therapy firm focused on pioneering logic-gated cell therapies for solid tumors, has reported the administration of the initial dose to the first participant in the Phase 1 clinical trial of A2B694. This multi-center Phase 1 dose-escalation study, known as EVEREST-2, aims to assess the safety and establish the recommended dose of A2B694 in patients with mesothelin-expressing solid tumors, such as ovarian cancer, mesothelioma, pancreatic cancer, colorectal cancer, and non-small cell lung cancer, who exhibit loss of HLA-A*02 expression.
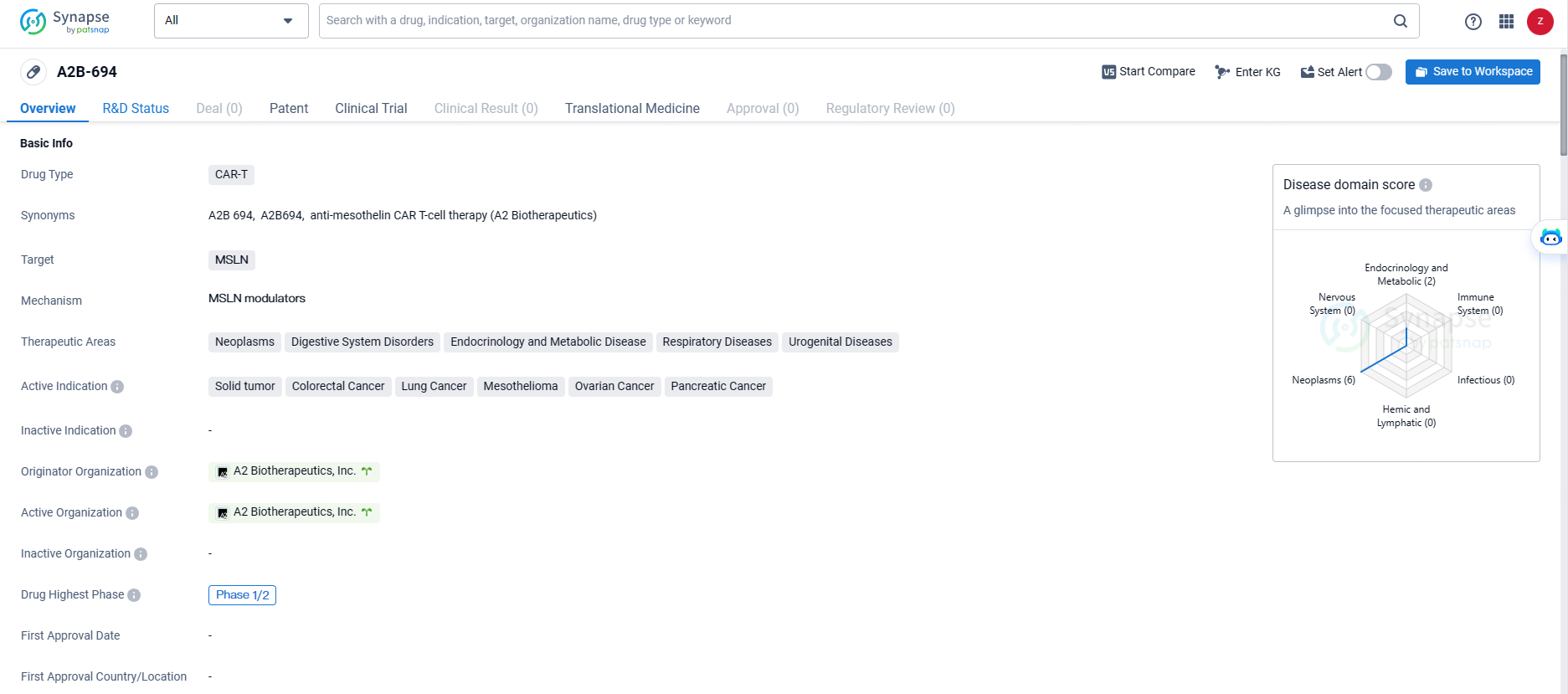
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
A2B694 is the second autologous cell therapy in clinical trials by A2 Bio employing its proprietary Tmod™ platform. This platform features a dual-receptor mechanism, including an activator that targets tumor cells and a blocker that safeguards normal cells. The dual-receptor system is designed to selectively eliminate tumor tissues expressing mesothelin while permanently lacking the HLA-A*02 gene. This innovative approach seeks to address a fundamental problem in solid tumor oncology—selectively exterminating cancer cells while preserving healthy ones.
"Administering the first dose to a patient in this study is a crucial advancement towards offering a precise, novel CAR T therapy for patients with mesothelin-expressing solid tumors who have no curative treatment options. Unfortunately, current treatments for these patients are palliative and limited by toxicity in recurrent, unresectable, locally advanced, or metastatic stages. All of us at A2 Bio extend our gratitude to the participating patients, investigators, and clinical care teams," commented Dr. William Go, chief medical officer of A2 Bio.
Beyond A2B694, A2 Bio is also progressing with the clinical development of A2B530 and other preclinical initiatives as it seeks to expand its pipeline using its proprietary Tmod™ platform.
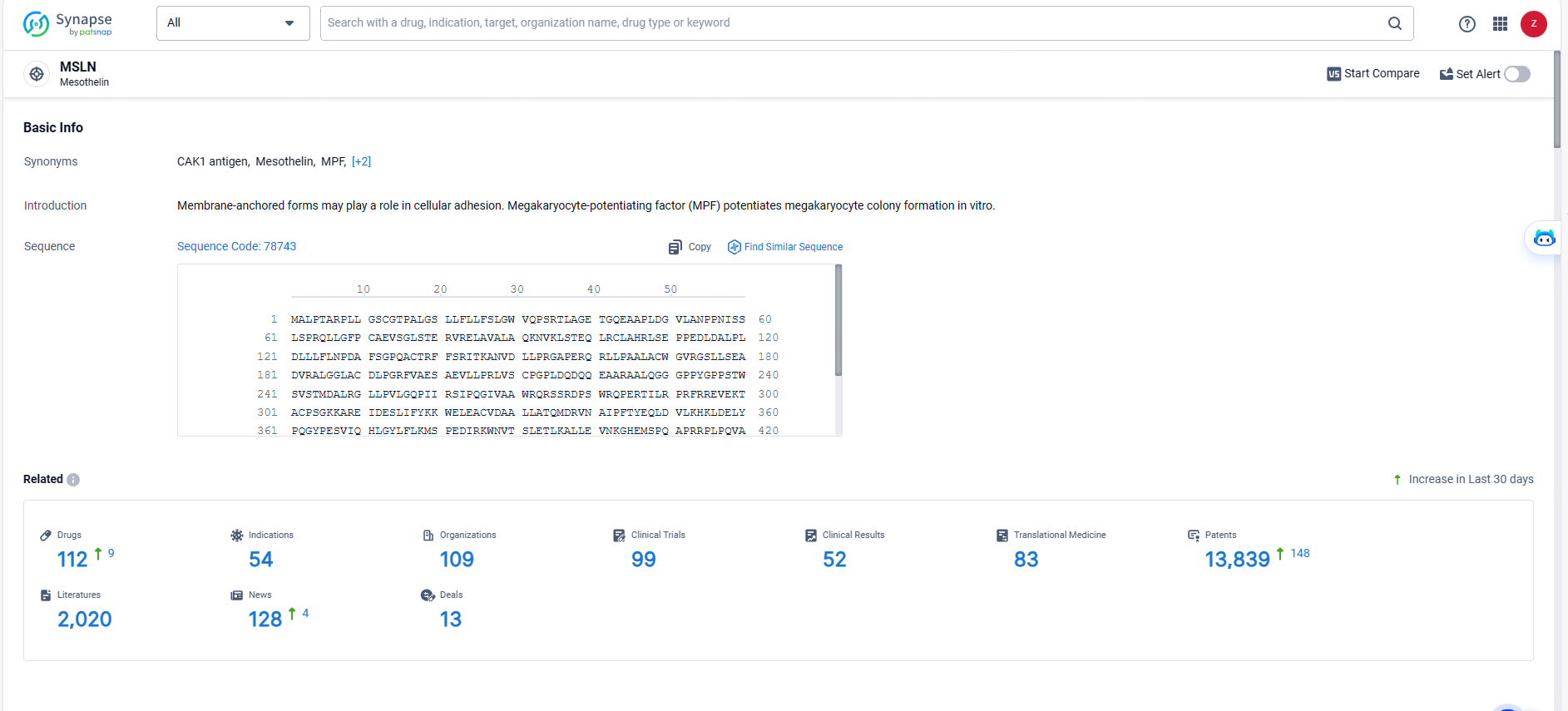
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 112 investigational drugs for the MSLN targets, including 54 indications, 109 R&D institutions involved, with related clinical trials reaching 99, and as many as 13839 patents.
A2B-694 targets the MSLN protein for the treatment of various cancers and related disorders. The drug has reached the Phase 1/2 stage of clinical development, indicating early promise but requiring further study to establish its potential as a new treatment option for patients.