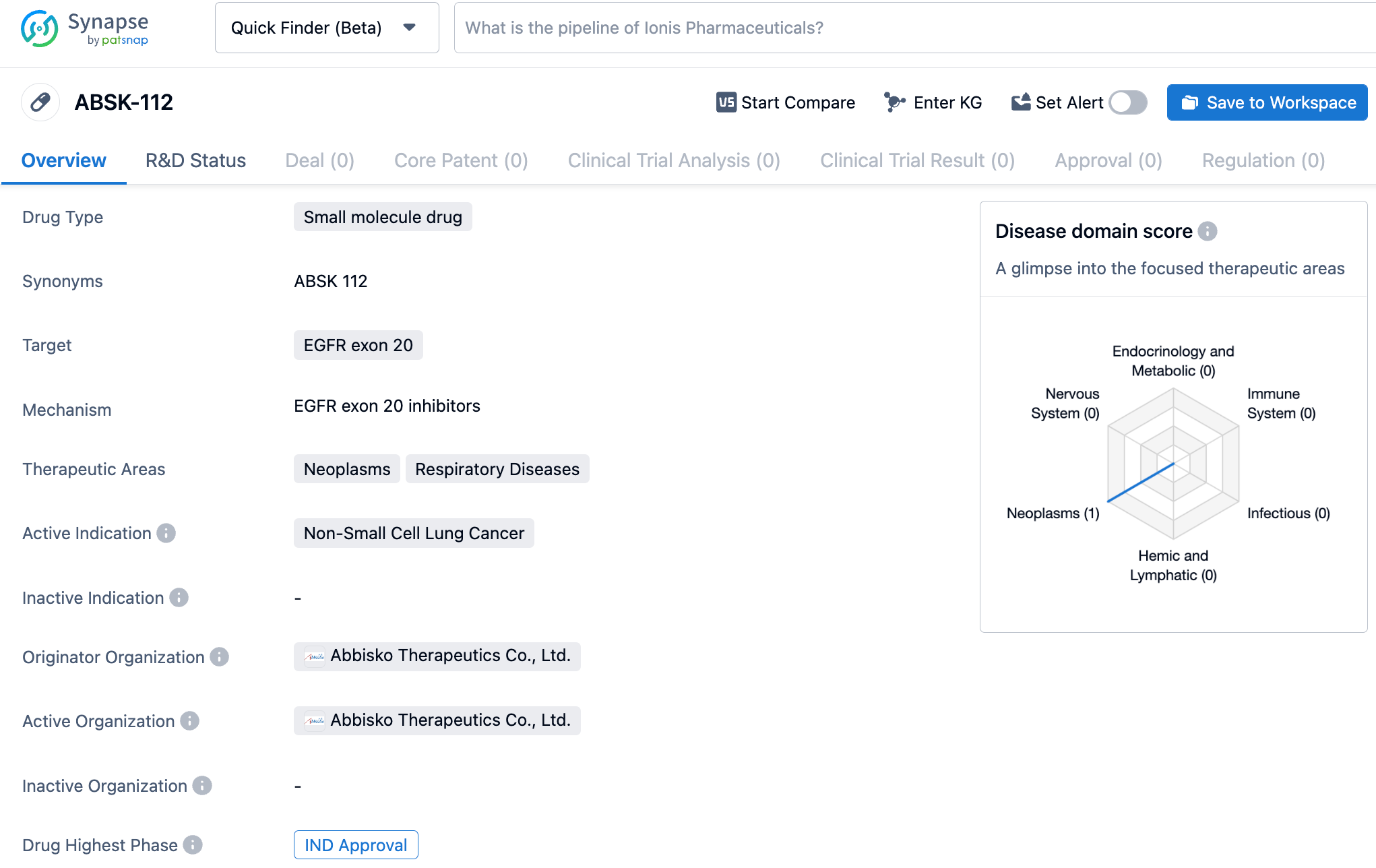
Abbisko Therapeutics' EGFR exon20ins inhibitor, ABSK112, has been approved for clinical use in China for the treatment of non-small cell lung cancer
Recently, Abbisko Therapeutics announced that its independently developed next-generation EGFR Exon20ins inhibitor ABSK112 has received approval from the National Medical Products Administration (NMPA) of China for clinical trial applications. This marks the initiation of China's first phase 1 human clinical trial targeting non-small cell lung cancer (NSCLC). Prior to this, ABSK112 had already received clinical research authorization from the US FDA, and phase 1 studies will be conducted simultaneously in China and the US.
ABSK112 is a second-generation EGFR Exon20ins oral inhibitor with excellent activity, selectivity, and brain penetration characteristics. Compared to previously marketed or clinical trial-stage EGFR Exon20ins inhibitors, ABSK112 demonstrated superior brain penetration, superior selectivity against wild-type EGFR, and broader coverage of Exon20ins mutations in preclinical studies. It showed positive in vivo efficacy in various mouse tumor models with EGFR Exon20ins mutations and has potential to achieve better safety and efficacy profiles in the clinic, ultimately becoming a new generation of highly effective drugs in its class.
According to a press release from Abbisko Therapeutics, the newly approved ABSK112 is set to undergo a first-in-human (FIH), multi-center, non-randomized, open-label phase 1 trial. The study will initially be conducted in NSCLC patients with escalating doses of ABSK112, with the goal of assessing the drug's safety, tolerance, pharmacokinetics (PK), and preliminary anti-tumor activity.
Lung cancer is the second most common cancer worldwide, with over 2 million new cases in 2020, including 227,875 in the US and 815,563 in China. Lung cancer has the highest mortality rate, accounting for over 1.7 million deaths annually, including 138,225 in the US (7.7 %) and 714,699 in China (39.8 %). NSCLC accounts for the largest proportion of all lung cancers (85%). Approximately 17% of lung adenocarcinomas in Caucasians and 50% in Asians have epidermal growth factor receptor (EGFR) mutations. By targeting NSCLC, an indication with a huge patient population, ABSK112 has enormous market potential once approved.
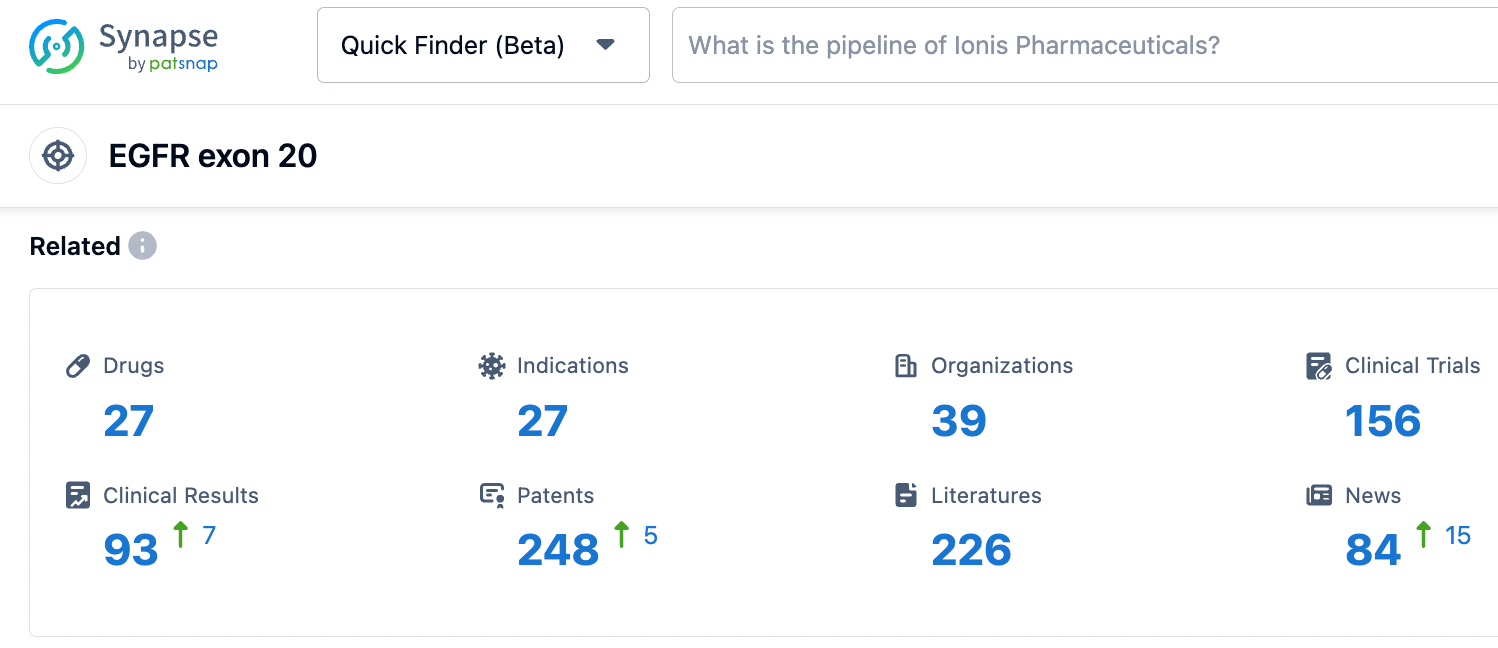
According to information disclosed by the Synapse database, as of October 28, 2023, there were 27 drugs under development targeting EGFR exon 20, with 27 indications, 39 research institutions involved, 156 related clinical trials, and as many as 247 patents. We look forward to the smooth progress of ABSK112 in its subsequent development, bringing new treatment options to patients.