AbbVie Reports EU Approval of SKYRIZI® (risankizumab) for Adult Ulcerative Colitis Treatment
AbbVie revealed that the European Commission has granted approval for SKYRIZI(risankizumab) to be used in adult patients suffering from moderately to severely active ulcerative colitis, especially those who did not respond adequately, lost response, or were intolerant to conventional or biologic therapies.
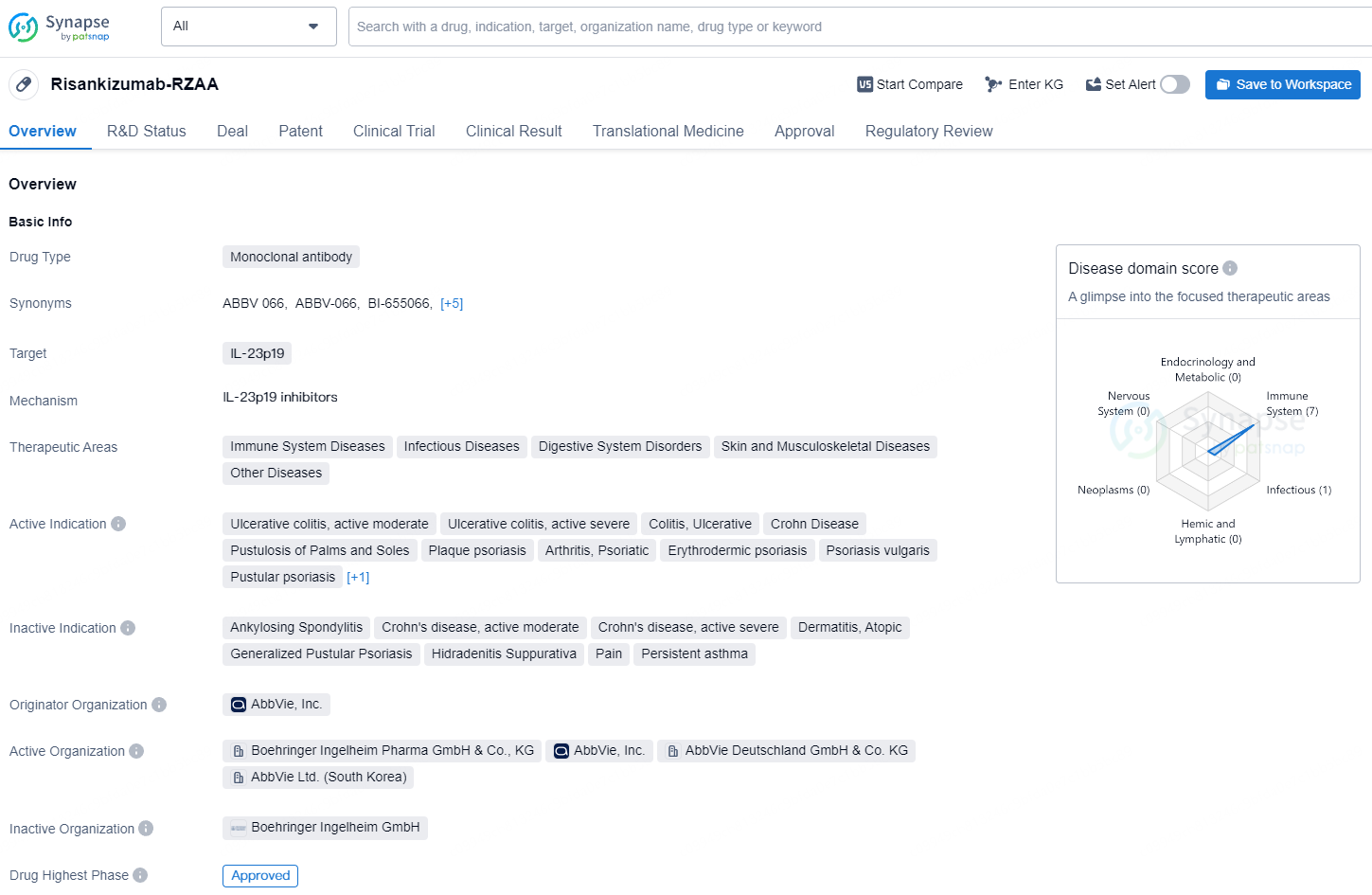
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Ulcerative colitis is a long-term, unpredictable, and at times incapacitating condition that requires continual symptom management for those affected,” stated Edouard Louis, M.D., Ph.D., professor and chief of gastroenterology at Liège University Hospital, as well as dean of the faculty at Liège University and investigator in the INSPIRE study. "In the INSPIRE and COMMAND trials, SKYRIZI resulted in significant enhancements in clinical remission and mucosal healing for patients.
These results are crucial since mucosal healing surpasses mere symptom control by restoring the intestinal lining, which correlates with better long-term outcomes. The recent approval offers a new therapeutic option to assist UC patients in achieving their long-term treatment objectives," he added.
UC is believed to impact approximately 5 million individuals globally, with its prevalence on the rise. Typical symptoms of UC include diarrhea, abdominal discomfort, blood in the stool, an urgent need to defecate, the passage of mucus from the rectum, and rectal pain and bleeding. The associated pain and discomfort often reduce patients' ability or willingness to engage in daily activities.
“The authorization of SKYRIZI for UC treatment equips doctors with a new proven treatment alternative for a diverse patient population with varied histories of previous treatments, including both conventional and biological therapies. Importantly, the Phase 3 trials demonstrated positive outcomes in mucosal healing, especially among those without prior experience with biologics or JAK inhibitor failures,” noted Roopal Thakkar, M.D., executive vice president of research & development and chief scientific officer at AbbVie.
For SKYRIZI induction, the advised dose is 1,200 mg administered via an intravenous infusion at week 0, week 4, and week 8. Beginning at week 12 and every 8 weeks following, the recommended maintenance dose is either 180 mg or 360 mg administered by subcutaneous injection, tailored to the individual patient's condition."
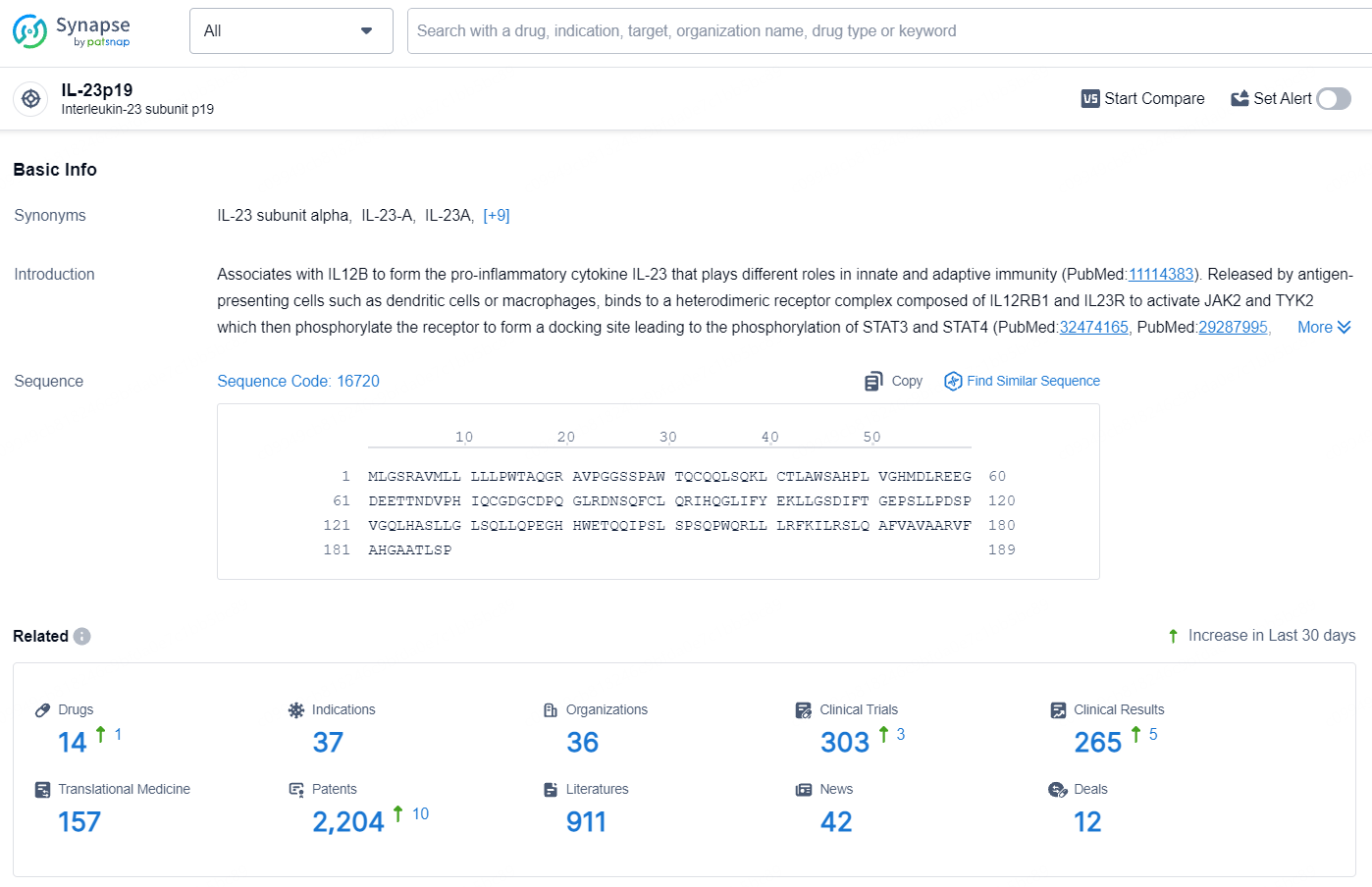
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 31, 2024, there are 14 investigational drugs for the IL-23p19 targets, including 37 indications, 36 R&D institutions involved, with related clinical trials reaching 303, and as many as 2204 patents.
Risankizumab-RZAA is a monoclonal antibody drug that targets IL-23p19 and is used in the treatment of a wide range of therapeutic areas, including immune system diseases. Its approval in Japan and NDA/BLA status in China demonstrate its potential to impact patients in multiple countries, and its orphan drug designation suggests it may address unmet medical needs in the treatment of certain diseases. As such, it represents a valuable contribution to the field of biomedicine and the ongoing efforts to improve patient care and outcomes.