AbbVie's Acquisition of Landos Biopharma Bolsters Inflammatory and Autoimmune Treatment Portfolio
AbbVie Inc. has entered into a formal arrangement to take over Landos Biopharma, Inc., an enterprise operating in the clinical phase specializing in the creation of innovative oral treatments targeting autoimmune conditions. The principal experimental compound in Landos' portfolio is NX-13, recognized as an inaugural agent in its class, functioning orally as an NLRX1 agonist. This compound exhibits a dual-action capability, reducing inflammation and promoting the restoration of epithelial tissue.
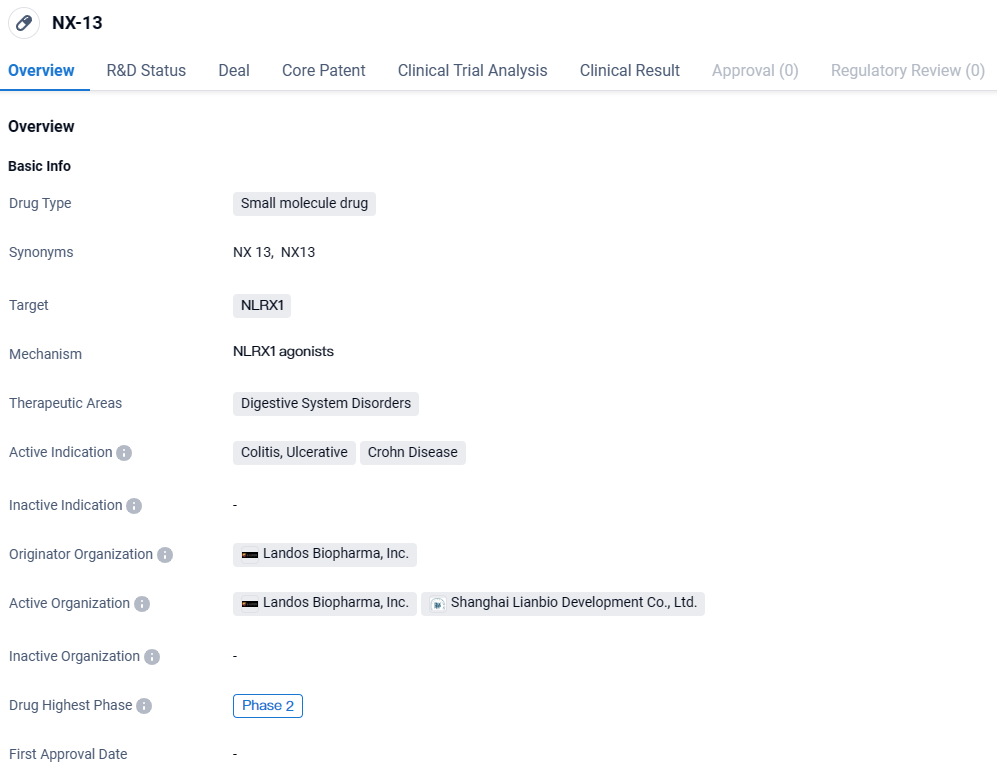
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Through this strategic purchase, our goal is to propel the progression of NX-13, an innovative, leading-edge, orally-administered compound with the capability to significantly improve the quality of life for individuals affected by ulcerative colitis and Crohn’s disease," stated Roopal Thakkar, M.D., the lead medical executive and a high-ranking vice president at AbbVie's global therapeutics division.
Gregory Oakes, at the helm of Landos as president and CEO, commented, "This declaration reflects the dedication and skill of the Landos team to our goal of innovating oral therapies that can fill an unmet medical need." "AbbVie's expertise in the therapeutics field and their exceptional global development capabilities position them ideally to further the progress of NX-13, which, with its dual-action mechanism of action, holds promise for a groundbreaking approach to treating ulcerative colitis and Crohn's disease."
The role of NLRX1 is pivotal in managing immunometabolism and the inflammatory response, playing a significant role in affecting various aspects of the pathology associated with inflammatory bowel disease. The NEXUS Phase 2 clinical trial, a randomized controlled study of NX-13 in ulcerative colitis, is actively recruiting participants across the U.S. and Europe.
Pursuant to the acquisition terms, AbbVie will take over Landos for $20.42 per share in an all-cash transaction at the completion of the deal, totaling approximately $137.5 million, followed by an additional conditional non-tradable payment right valued at up to $11.14 per share, summing to roughly $75 million more, contingent on hitting a specified clinical progression target. Anticipated to conclude in the second quarter of 2024, this transaction hinges on standard conditions for closure, including endorsement from Landos shareholders.
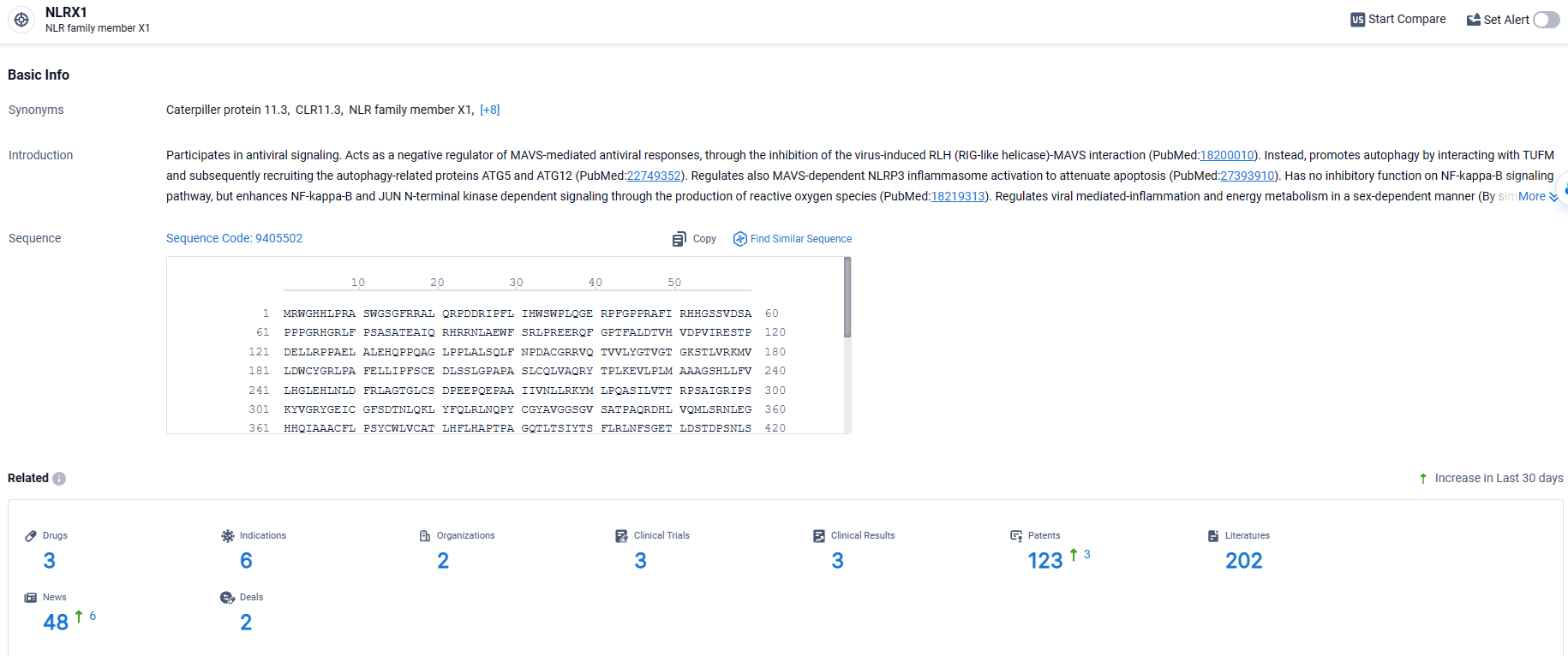
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 26, 2024, there are 3 investigational drugs for the NLRX1 target, including 6 indications, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 123 patents.
NX-13 is a small molecule drug being developed for the treatment of digestive system disorders, specifically colitis, ulcerative colitis, and Crohn's disease. The drug has reached Phase 2 of clinical development globally and Phase 1 in China. This indicates that NX-13 has shown promising results in early testing and is progressing towards further evaluation in larger patient populations.