Abdera Therapeutics Receives FDA Approval for IND Application of ABD-147
Abdera Therapeutics Inc., a biopharmaceutical firm utilizing its innovative antibody engineering ROVEr™ platform to create and develop customizable, precision radiopharmaceuticals aimed at cancer treatment, has revealed that the U.S. Food and Drug Administration has approved their Investigational New Drug application for ABD-147. This compound is the first radiopharmaceutical targeting delta-like ligand 3 (DLL3) designed for treating small cell lung cancer and large cell neuroendocrine carcinoma. Abdera anticipates starting a Phase 1 clinical trial in the latter half of 2024.
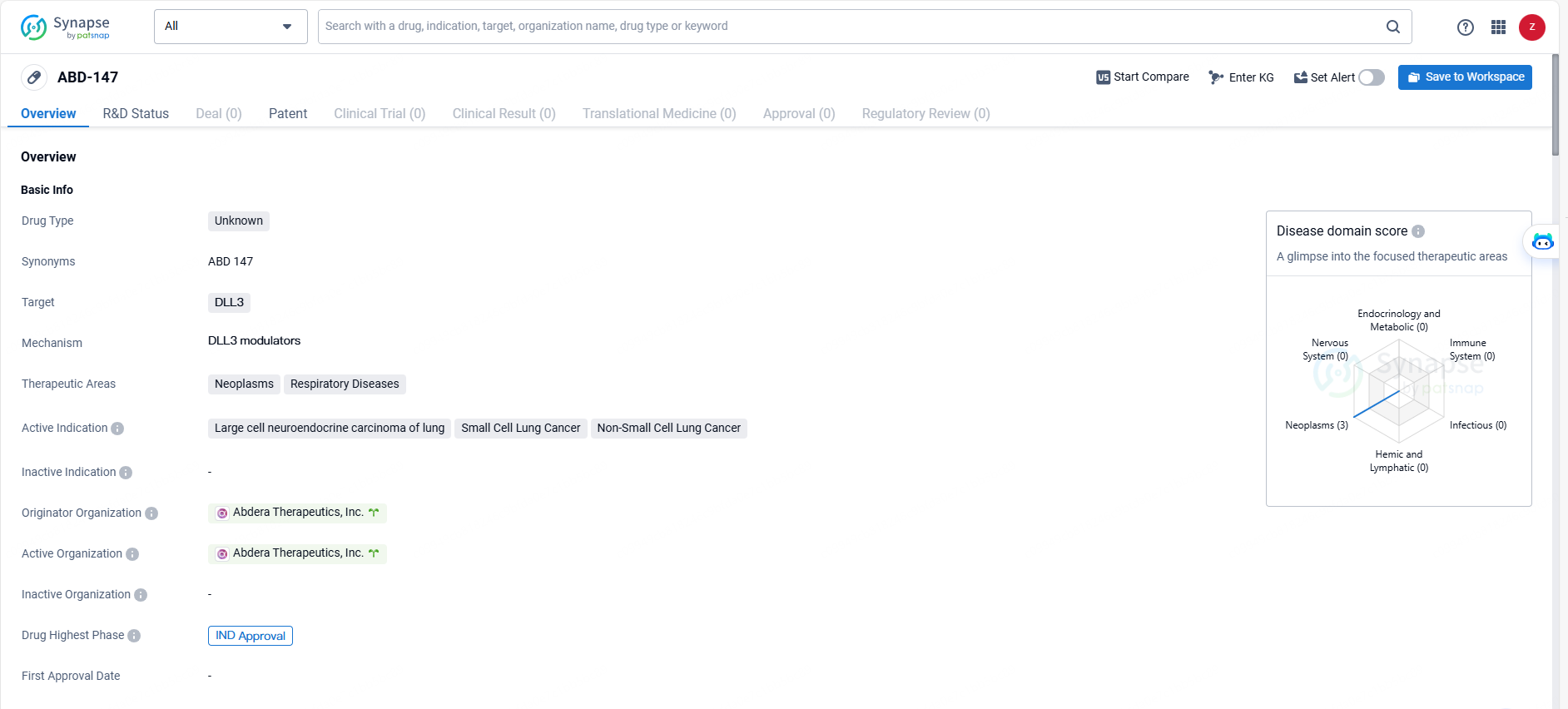
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ABD-147 is an advanced precision radiopharmaceutical biologic designed to deliver Actinium-225, a powerful alpha-emitting radioisotope, directly to solid tumors that express DLL3. DLL3 is a protein that appears on the surface of neuroendocrine tumors but is rarely found on normal cells or tissues.
"ABD-147 has the potential to be a leading treatment for SCLC and other aggressive neuroendocrine tumors," remarked Philippe Bishop, M.D., the chief medical officer. "Using our ROVEr platform, we have engineered ABD-147 with optimized pharmacokinetic properties and tumor penetration capabilities, aiming to eliminate tumor cells while minimizing radiation exposure to healthy tissues. We are optimistic that this potent next-generation radiotherapeutic will offer a significant breakthrough in addressing the medical needs for treating SCLC and other high-grade neuroendocrine cancers. We are excited to begin a Phase 1 clinical trial of ABD-147 later this year."
The Phase 1, first-in-human, open-label study is set to evaluate the safety and initial effectiveness of 225Ac-ABD-147 in patients with SCLC or LCNEC who have previously undergone platinum-based therapies. The study’s goal is to identify the recommended dosing regimen for further development.
"Receiving clearance from the FDA for our first IND is a pivotal achievement for Abdera as we evolve into a clinical-stage radiopharmaceutical company," stated Lori Lyons-Williams, president and CEO. "We believe our ROVEr™ platform sets the stage for a new era of innovation in targeted radiotherapeutics, with ABD-147 leading a strong pipeline of custom-engineered treatments we are moving into clinical trials."
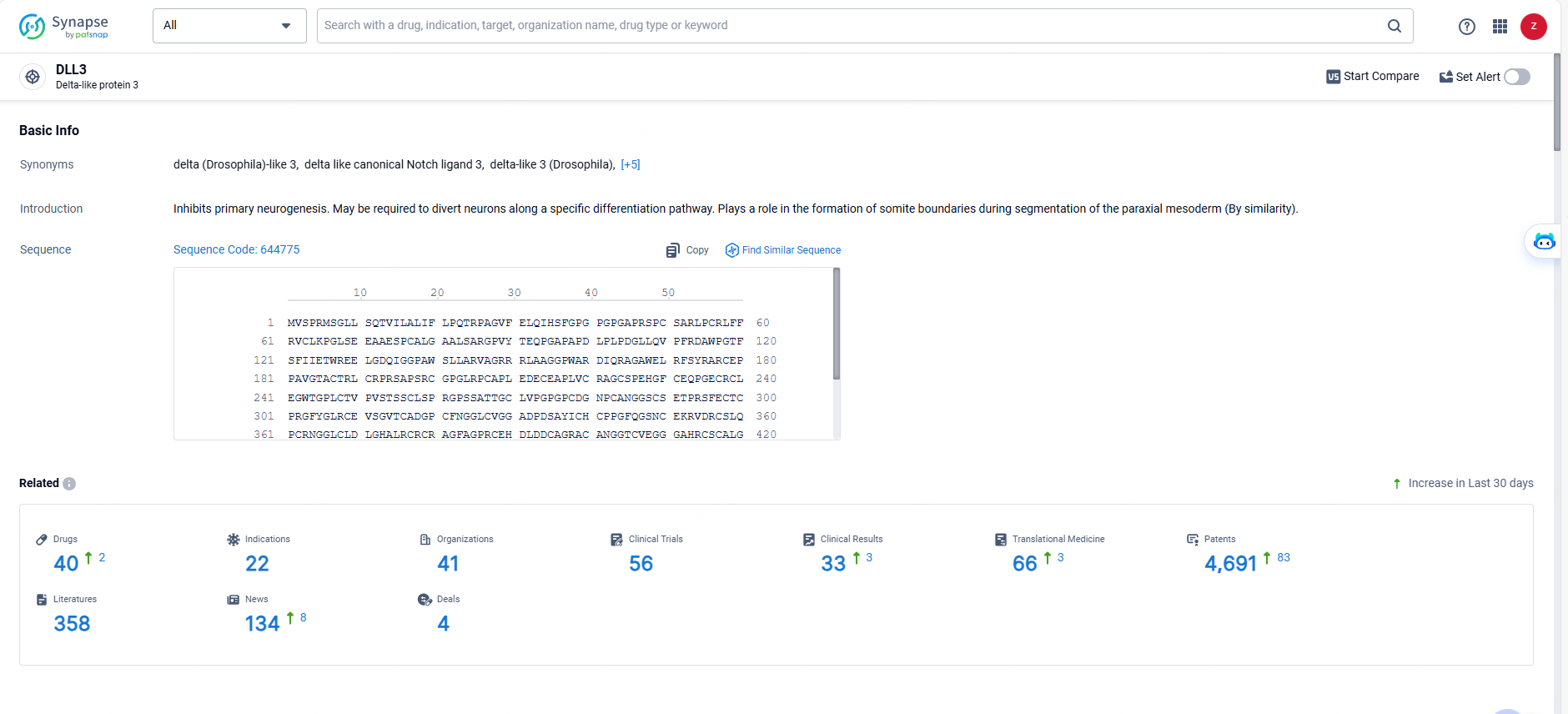
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 29, 2024, there are 40 investigational drugs for the DLL3 target, including 22 indications, 41 R&D institutions involved, with related clinical trials reaching 56, and as many as 4691 patents.
ABD-147 is a drug developed by Abdera Therapeutics, Inc. with a focus on targeting DLL3 and addressing neoplasms and respiratory diseases, particularly in the context of lung cancer. The drug has received IND approval, signifying its progression to clinical development and potential for further evaluation in treating the specified indications. Further research and clinical trials may provide additional insights into the efficacy and safety of ABD-147 in the treatment of these conditions.