AbelZeta Presents Initial C-CAR168 Findings for Autoimmune Treatment at ACR 2024
AbelZeta Pharma, Inc., a worldwide biopharmaceutical enterprise in the clinical research phase, is dedicated to the innovation and advancement of exclusive cell-based therapeutic products. it was announced that preclinical findings from the C-CAR168 trial were shared as a podium presentation at the American College of Rheumatology (ACR) Convergence 2024, which took place in Washington, DC, from November 14-19, 2024. C-CAR168 represents a groundbreaking autologous bi-specific CAR-T therapy designed to target both CD20 and B-cell maturation antigen (BCMA) for patients suffering from resistant and refractory Lupus Nephritis (LN) and Systemic Lupus Erythematosus (SLE). In May 2024, the Food and Drug Administration (FDA) approved the Investigational New Drug (IND) application for advancing C-CAR168 into Phase 1 clinical trials.
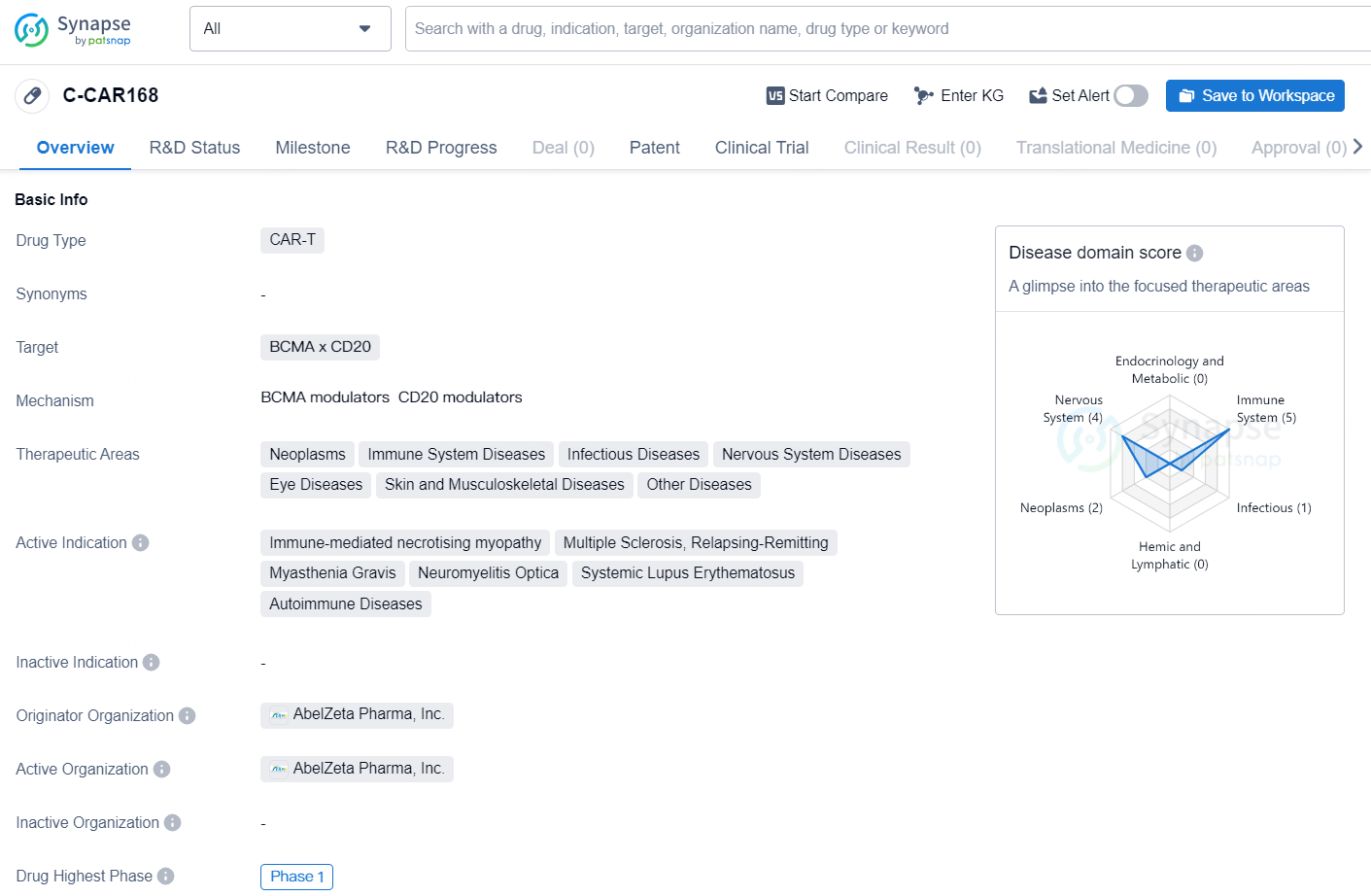
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Key Highlights:
Scientific Basis for Targeting B Cells and Plasma Cells: C-CAR168 has been designed to specifically target CD20 and BCMA, which are markers linked to pathogenic B cells and long-lived plasma cells (LLPCs) that play a role in autoimmune phenomena. Research indicates that C-CAR168 successfully triggers cell death in cells that are positive for CD20 and/or BCMA, including the age-associated B cell (ABC) subset, which is notably prevalent in autoimmune disorders.
Strong In Vivo Cytotoxicity: In studies using immunodeficient mouse models, a single administration of C-CAR168 displayed significant efficacy across various doses, effectively suppressing the proliferation of cells that are either single-positive or double-positive for CD20 and BCMA in numerous xenograft experiments.
Minimal Off-Target Toxicity: Extensive testing, including membrane proteome analysis, has verified that C-CAR168 specifically targets CD20 and BCMA proteins, showing no significant affinity for non-target cells.
Safety Assessment: In vitro studies revealed a low probability of carcinogenic effects or unintended cell transformation, indicating the potential safety of C-CAR168 for therapeutic use.
Clinical Progress for C-CAR168: In an investigator-led trial in China involving patients with refractory/relapsing LN, 7 participants received both a suboptimal and a potentially optimal dose. All patients demonstrated depletion of B cells and plasma cells in their bloodstream, alongside a favorable safety profile and early positive clinical indicators at the suboptimal dose after at least 3 months of monitoring.
Future Indications: The team is actively exploring additional indications such as Neuromyelitis Optica Spectrum Disorder (NMOSD), Immune-Mediated Necrotizing Myopathy (IMNM), and Systemic Sclerosis (SSc), in preparation for global trials expected to commence around mid-2025.
US IND Submission: The submission for the US Investigational New Drug application was approved on May 31, 2024.
“We are pleased with the advancements we have made concerning C-CAR168 to date. We believe it holds promise as an exceptional treatment for patients enduring diverse autoimmune disorders with severe symptoms and poor prognoses,” stated Tony (Bizuo) Liu, Chairman and CEO. “This progress signifies a significant advancement towards providing potentially best-in-class and/or best-in-disease innovative cell therapies for cancer, as well as inflammatory and immunological disorders.”
Yihong Yao, PhD, Chief Scientific Officer of AbelZeta, remarked, “We are enthusiastic about the prospects of C-CAR168. CD20 has demonstrated effectiveness as a target for B cell elimination in the treatment of relapsed/refractory non-Hodgkin lymphoma (NHL). It also shows promise in autoimmune diseases, as seen with other approved treatments. Our dual-target CAR-T therapies targeting CD20 and BCMA have proven to be both safe and effective in NHL and multiple myeloma. C-CAR168 was developed to identify and eliminate B cells, plasmablasts, and LLPCs, thereby addressing the source of autoantibodies and offering potential definitive treatment for severe autoimmune conditions that resist existing therapies, including LN and SLE.”
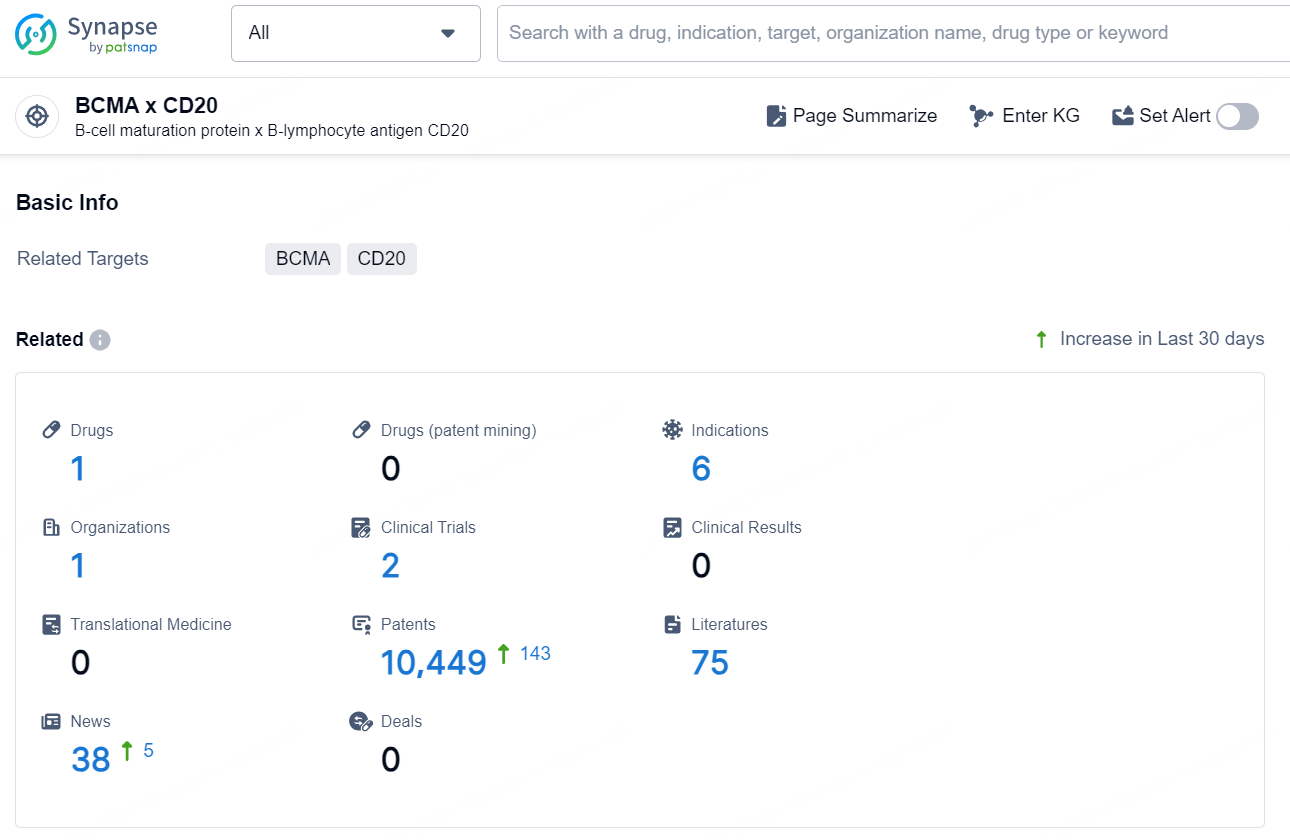
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 1 investigational drug for the BCMA and CD20 target, including 6 indications, 1 R&D institution involved, with related clinical trials reaching2, and as many as 10449 patents.
C-CAR168 is a CAR-T drug developed by AbelZeta Pharma, Inc. It targets BCMA and CD20 and falls under the category of CAR-T therapy. The drug has a broad range of therapeutic areas including neoplasms, immune system diseases, infectious diseases, nervous system diseases, eye diseases, skin and musculoskeletal diseases, and other diseases.