Accord Healthcare Gains Approval for IMULDOSA®, a Stelara® Biosimilar
Accord has revealed that the European Commission (EC) has approved the marketing of Imuldosa® (development code: DMB-3115), a biosimilar of Stelara®, which is intended for various immune-mediated inflammatory conditions. This EC approval comes after a favorable opinion was provided on 19 October 2024 by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA), and it is valid across all 27 Member States of the European Union (EU) as well as Iceland, Norway, and Liechtenstein.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The positive opinion from the CHMP is founded on a thorough array of analytical, non-clinical, and clinical similarity evidence, which includes a multi-regional Phase III clinical trial focused on patients with plaque psoriasis. This research demonstrated that DMB-3115 and Stelara® were therapeutically equivalent in the primary endpoint, with a safety profile that is also comparable.
The marketing authorization facilitates the entry of Imuldosa® into the European market for ustekinumab, which is estimated to be worth around €2.9 billion (approximately US$3.18 billion) as reported by IQVIA MAT data from June 2024.
Intas has obtained exclusive rights to market Imuldosa® globally, with the exception of Japan, Korea, and a few other Asian regions. On October 10, 2024, Imuldosa® received approval from the U.S. FDA.
Accord Healthcare Limited (Accord) has announced that the European Commission (EC) has approved Imuldosa® (development code: DMB-3115), a biosimilar of Stelara® (ustekinumab), which is marketed by Janssen Biotech Inc., a subsidiary of Johnson & Johnson.
Ustekinumab is a human monoclonal antibody that inhibits the cytokines interleukin-12 and interleukin-23, both of which play crucial roles in inflammatory and immune responses. Stelara® is approved for a variety of immune-mediated inflammatory conditions and has reported total global revenue of US$19 billion, with US$3.2 billion generated from Europe, according to IQVIA MAT data from June 2024.
Joe Dunford, VP of Specialty Brands, remarked, “Accord is dedicated to becoming a key figure in the autoimmune treatment sector. We are pleased that the European Commission (EC) has approved our fifth biosimilar in Europe, Imuldosa®. This authorization guarantees that patients have access to effective therapies in Europe and beyond. We are committed to furthering our biosimilar portfolio, aiming to introduce 20 biosimilars by 2030.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
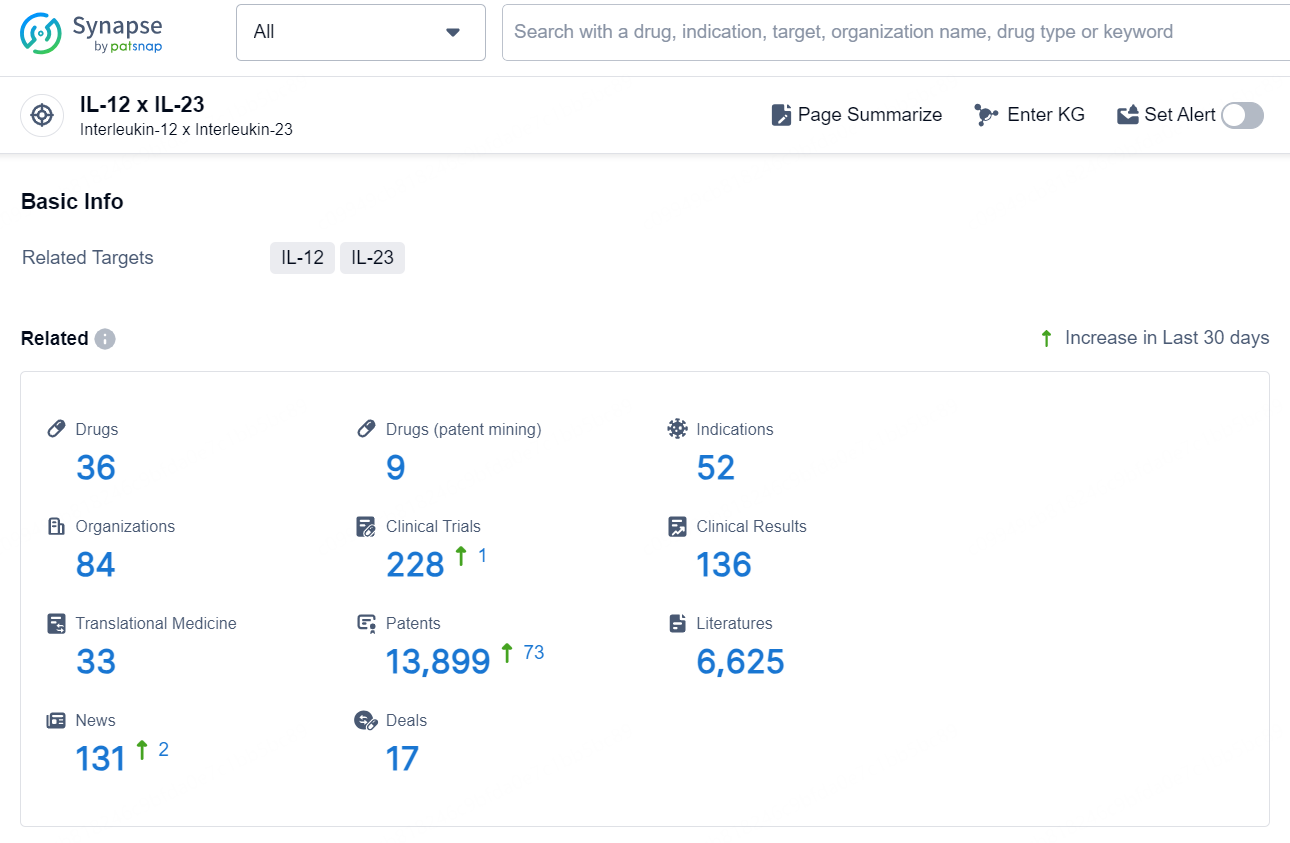
According to the data provided by the Synapse Chemical, As of December 20, 2024, there are 36 investigational drugs for the IL-12 x IL-23 target, including 52 indications, 84 R&D institutions involved, with related clinical trials reaching 228, and as many as 13899 patents.
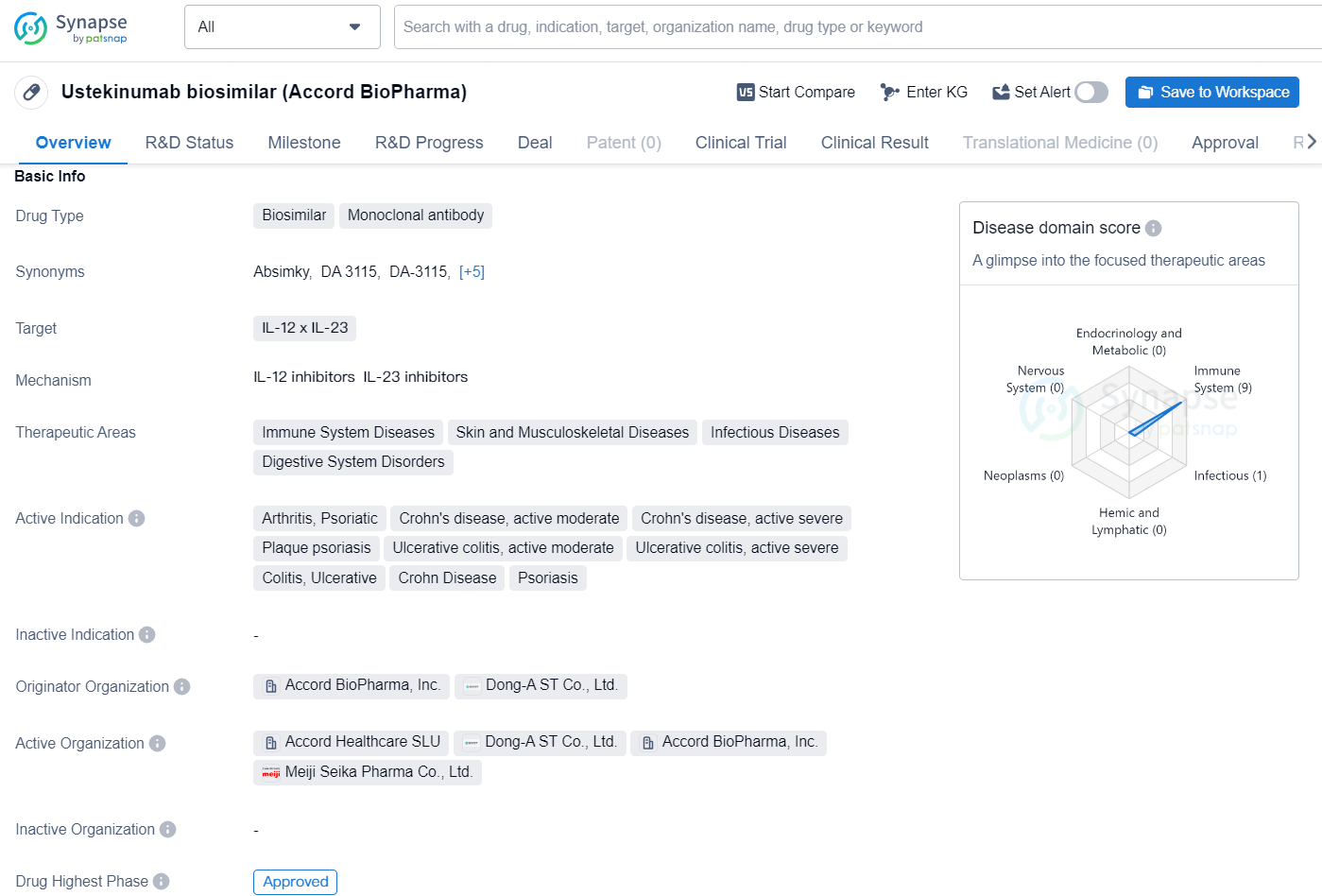
The Ustekinumab biosimilar (Accord BioPharma) is a monoclonal antibody drug type that targets the IL-12 x IL-23 pathways. It is indicated for a range of therapeutic areas, including immune system diseases, skin and musculoskeletal diseases, infectious diseases, and digestive system disorders. The active indications for this drug include arthritis, psoriatic, Crohn's disease (moderate and severe), plaque psoriasis, ulcerative colitis (moderate and severe), and colitis, ulcerative, as well as Crohn Disease and Psoriasis.