Acousia Therapeutics Begins Enrollment for Phase II of PROHEAR Trial
Acousia Therapeutics GmbH, a clinical stage biotechnology enterprise rooted in Tübingen and dedicated to improving and conserving naturally occurring auditory function, recently disclosed the inaugural randomization of a participant for its PROHEAR-Study.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The investigational PROHEAR-Study, consisting of a double-blind, Phase 2a trial with a contralateral comparison framework, is examining the protective effects on hearing function of ACOU085 in individuals with testicular germ-cell tumors who are being treated with high dosage cisplatin chemotherapy protocols (cis-Pt ≥300 mg/m^2).
Testicular cancer originating from germ cells is the predominant malignancy affecting adolescent and young adult males, showing an upward trend in occurrence rates. Following the adoption of cisplatin in cancer management protocols in the latter half of the 20th century, the survival rate for individuals with metastatic testicular cancer has greatly improved, offering life expectancies extending between 30 to 60 years post-treatment. One of the primary adverse effects of cisplatin is ototoxicity, characterized by permanent injury to the sensory hair cells within the inner ear, specifically the external hair cells.
Although hearing loss has been regarded as an inescapable consequence of cancer survival in the past, it imparts substantial quality of life detriments on patients including challenges in communication, increased tiredness, decreased social engagement, and a heightened propensity for cognitive decline and dementia later in life. ACOU085 has shown promising results in preclinical tests to decrease ototoxicity linked with cisplatin and shield the sensory cells in the ear from such damage.
In the methodically structured PROHEAR-Study, participants inflicted with testicular cancer are administered intratympanic injections of ACOU085 in one ear, while the other ear receives a saline solution as a placebo before each chemotherapy session. This setup allows each individual to act as their own comparator for the study's outcomes. This important trial is being carried out at top-tier medical university centers throughout Germany, with full support from the Study Group of the German Society of Otorhinolaryngology.
Dr. Tim Bölke, CEO and CMO of Acousia Therapeutics, expressed enthusiasm about the progression of their clinical program: "Admitting the inaugural patient into our Clinical Phase 2 project for ACOU085 signals another pivotal milestone in our journey to address both acute and chronic variants of auditory loss."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
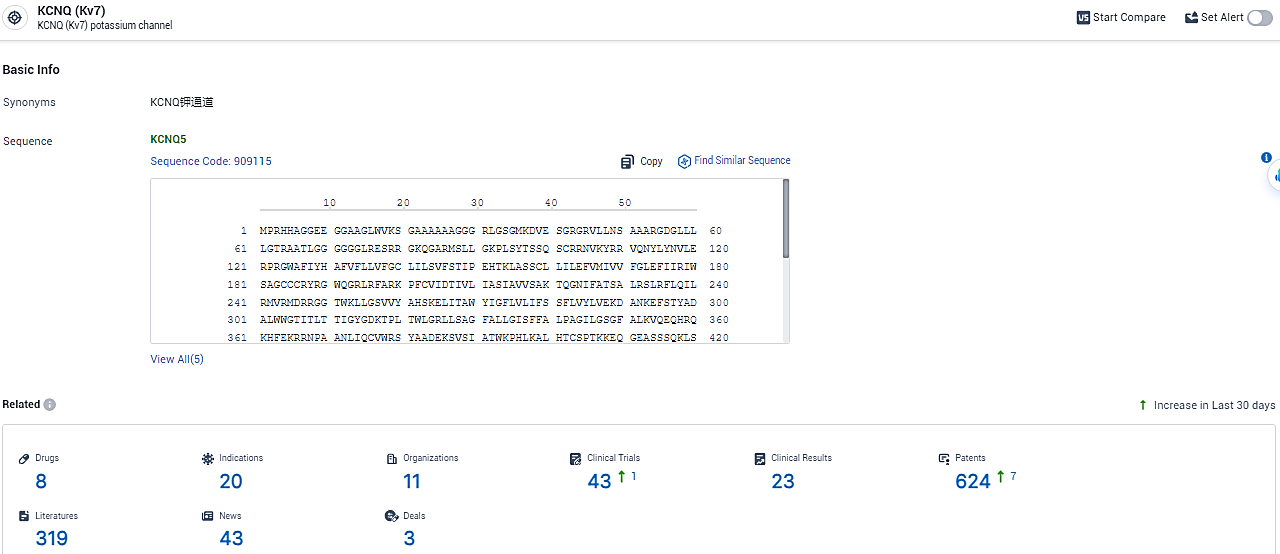
According to the data provided by the Synapse Database, As of February 22, 2024, there are 8 investigational drugs for the KCNQ target, including 20 indications, 11 R&D institutions involved, with related clinical trials reaching 43, and as many as 624 patents.
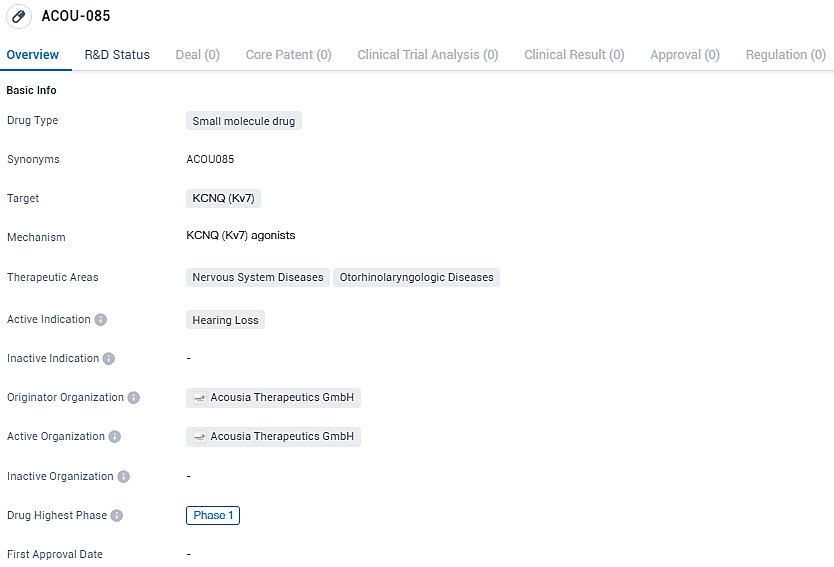
ACOU-085 targets KCNQ and aims to address hearing loss, a condition falling under the therapeutic areas of Nervous System Diseases and Otorhinolaryngologic Diseases. Currently in Phase 1, ACOU-085 holds promise as a potential treatment option for individuals suffering from hearing loss.