Advances in Clinical Research on PPAR Agonists
PPAR, or peroxisome proliferator-activated receptor, is a group of nuclear receptor proteins found in the human body. These receptors play a crucial role in regulating various physiological processes, including metabolism, inflammation, and cell differentiation.
PPARs, as a nuclear hormone receptor family, are ligand-activated intranuclear transcription factors, composed of three homologous members: PPARα, PPARβ/δ, and PPARγ. Among them, PPARα primarily regulates the transport, esterification, and oxidation of fatty acids. PPARβ/δ is majorly associated with the oxidation of fatty acids and glucose uptake. PPARγ primarily controls the differentiation of adipocytes, lipid storage, and insulin sensitivity.
PPARs act as transcription factors, meaning they can influence gene expression by binding to specific DNA sequences. They are involved in lipid and glucose metabolism, making them important targets for pharmaceutical interventions in conditions like diabetes, obesity, and cardiovascular diseases. PPARs also have anti-inflammatory properties and are implicated in regulating immune responses. Understanding the role of PPARs is essential for developing drugs that can modulate these receptors and potentially treat various diseases.
PPAR Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 277 PPAR drugs worldwide, from 281 organizations, covering 180 indications, and conducting 1926 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.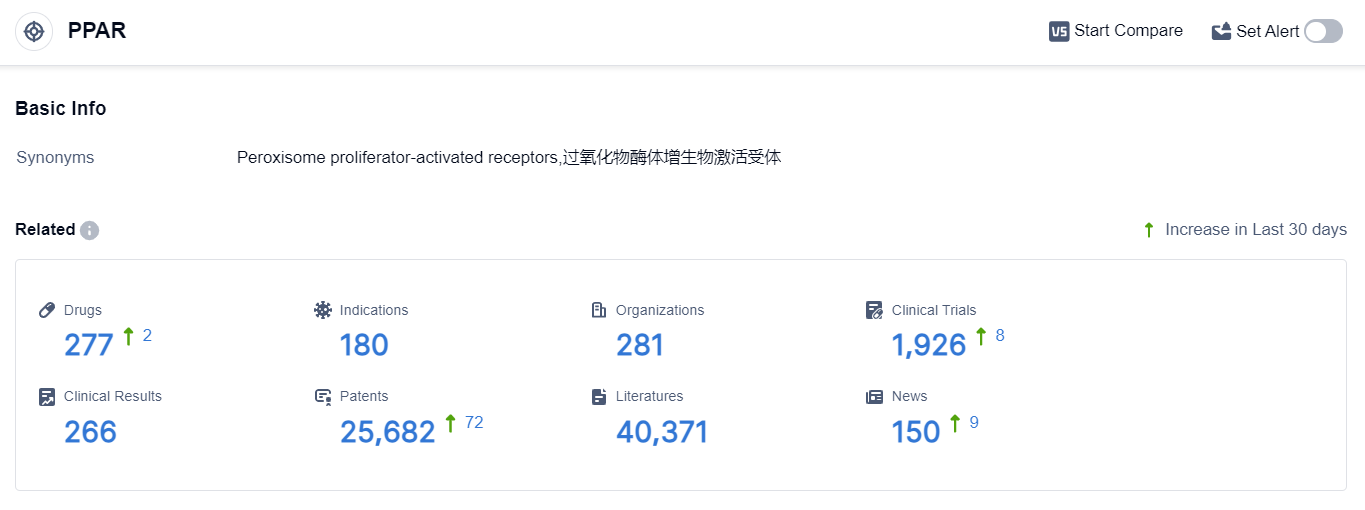
The analysis of the target PPAR reveals a competitive landscape with multiple companies actively involved in research and development. Takeda Pharmaceutical Co., Ltd., Viatris Inc., and GSK Plc are among the companies with the highest number of approved drugs, indicating their strong R&D progress.
The indications with the highest number of approved drugs are hyperlipidemias and diabetes mellitus type 2, suggesting a focus on these areas. Small molecule drugs are the most rapidly progressing drug type, indicating their significance in PPAR-targeted drug development.
China, the European Union, Japan, and the United States are the countries/locations with the highest number of approved drugs, with China showing significant progress. Overall, the target PPAR presents a competitive landscape with a focus on specific indications and drug types, and China emerges as a key player in the development of PPAR-targeted drugs.
The World's First PPAR Agonist:Chiglitazar Sodium
Chiglitazar Sodium is a small molecule drug that falls under the category of biomedicine. It primarily targets PPAR. The drug is designed to address various therapeutic areas, including digestive system disorders and endocrinology and metabolic diseases.
Chiglitazar Sodium has shown potential in treating specific indications, such as Diabetes Mellitus, Type 2, and Nonalcoholic Steatohepatitis. These conditions are prevalent worldwide and pose significant health challenges. By targeting PPAR, Chiglitazar Sodium aims to regulate glucose and lipid metabolism, potentially improving the management of these diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug was developed by Shenzhen Chipscreen Biosciences Co., Ltd., an originator organization in the pharmaceutical industry. It has undergone rigorous clinical trials and has achieved the highest phase of approval in China. This indicates that the drug has successfully met the necessary regulatory requirements and demonstrated its safety and efficacy in treating the targeted indications.
Chiglitazar Sodium received its first approval in China in October 2021. This signifies a significant milestone for the drug and its potential impact on patients in the country. However, it is important to note that the drug's approval is currently limited to China, and its availability in other countries may vary.
The approval of Chiglitazar Sodium highlights the continuous efforts in the field of biomedicine to develop innovative treatments for complex diseases. The drug's focus on PPAR as a therapeutic target reflects the growing understanding of the molecular mechanisms underlying various disorders.
In conclusion, Chiglitazar Sodium is a small molecule drug developed by Shenzhen Chipscreen Biosciences Co., Ltd. It targets PPAR and aims to address digestive system disorders and endocrinology and metabolic diseases. With its approval in China, the drug holds promise in treating Diabetes Mellitus, Type 2, and Nonalcoholic Steatohepatitis. Its successful development and approval underscore the ongoing advancements in the pharmaceutical industry to improve patient outcomes in the field of biomedicine.
Pemafibrate
Pemafibrate is a small molecule drug that targets PPARα, a receptor involved in regulating lipid metabolism. It is primarily used in the treatment of various disorders related to the digestive system, endocrinology, and metabolic diseases. The drug has been approved for use in several indications, including hyperlipidemias, hypercholesterolemia, dyslipidemias, hypertriglyceridemia, nonalcoholic steatohepatitis, liver cirrhosis, biliary, and primary biliary cholangitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Pemafibrate was developed by Kowa Co., Ltd., a pharmaceutical company based in Japan. It received its first approval in July 2017 in Japan, making it available for use in the country. The drug has also undergone clinical trials in China, reaching phase 3, which indicates that it is in an advanced stage of development in the Chinese market.
One notable aspect of Pemafibrate is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the drug's manufacturer, such as extended market exclusivity and financial support for research and development.
Overall, Pemafibrate represents a significant advancement in the field of biomedicine, particularly in the treatment of lipid-related disorders. Its approval in Japan and ongoing clinical trials in China suggest that it has shown promising results in terms of safety and efficacy. As a small molecule drug targeting PPARα, it offers a potential therapeutic option for patients suffering from various digestive system disorders, endocrinology, and metabolic diseases.






