AI Dominates Nobel Prizes: What Does This Mean for Drug Discovery?
This year's Nobel Prize announcements saw a sweeping victory for Artificial Intelligence (AI).
First, on October 8, the Nobel Prize in Physics was awarded to two pioneers in AI: John J. Hopfield, a professor at Princeton University, and Geoffrey E. Hinton, a professor at the University of Toronto, in recognition of their "foundational discoveries and inventions in machine learning with artificial neural networks." Geoffrey Hinton, the 2018 Turing Award winner, is regarded as a giant in deep learning and is often referred to as the "Godfather of AI." OpenAI co-founder and former chief scientist Ilya Sutskever was once a student of Geoffrey Hinton.
Following this, on October 9, the Nobel Prize in Chemistry was announced. Half of the award went to David Baker "for his contributions to computational protein design," while the other half was jointly awarded to Demis Hassabis and John M. Jumper "for their achievements in protein structure prediction." Heiner Linke, Chair of the Nobel Chemistry Committee, noted that the two discoveries recognized by the 2024 Nobel Prize in Chemistry have opened up endless possibilities in biochemistry. Baker achieved the almost impossible feat of designing entirely new types of proteins, while Hassabis and Jumper, through their AI models, realized a 50-year-old dream—predicting complex protein structures.
AI Technology Booming, AI + Pharma Expected to Become the Next Golden Track
AI technology, through machine learning, deep learning, and reinforcement learning, has already played a significant role throughout the entire pharmaceutical process, from drug discovery and preclinical research to clinical trials. Since early exploration in the 1990s, AI in pharma has gone through periods of technological accumulation, validation, and rapid development. It is now at a stage of active technological innovation, evident policy support, and vast market potential. According to Precedence Research, the AI pharmaceutical industry is expected to experience high growth over the next decade. The market size reached $1.17 billion in 2023 and is projected to exceed $11.8 billion by 2032, with a CAGR of 29.3% from 2023 to 2032.
Analysis of the AI Pharma Industry Chain
The AI pharma industry chain can be divided into upstream, midstream, and downstream segments.
The upstream involves computing power, algorithms, and data, primarily split into two categories: companies providing AI technology and companies providing biotechnology. Companies offering AI technology supply auxiliary pharmaceutical hardware such as servers and chips, as well as software, including various machine learning, deep learning, and other AI algorithms, along with data collection and processing platforms, open-source software packages, and cloud computing platforms. Biotech companies include those providing CRO (Contract Research Organization) services and advanced equipment.
The midstream segment is mainly categorized into four groups: AI+biotech, AI+CRO, AI+SaaS, and the involvement of major IT companies in AI pharma.
AI+biotech: Classified by drug properties or therapeutic methods, it can be further divided into three subcategories: small molecule drugs, large molecule drugs, and cell and gene editing methods.
AI+CRO: In this category, AI assists clients in better delivering lead compounds or potential clinical candidates (PCCs), which pharmaceutical companies then develop further or advance through partnerships.
AI+SaaS: This involves providing AI-assisted drug development platforms to empower companies, accelerating research and development processes while saving time and costs.
Major IT companies: Participate in the sector through external investments, building proprietary platforms, and offering computing power and computational frameworks.
The downstream segment of the AI pharma industry chain includes traditional pharmaceutical companies and CROs. Traditional pharma companies enter the AI pharma track by building in-house teams, making external investments, or collaborating with CROs and technology firms. CROs enter the space through venture capital investments, building internal algorithm teams, adopting external AI technologies, or partnering with AI pharma companies.
AI+Pharma Technology on the Rise, Diverse Applications Flourishing
New drug development is characterized by long cycles, high investments, and significant risks. AI's involvement across the entire drug development process accelerates the speed of drug discovery. For example, in the case of small molecule drugs, the development cycle typically takes around 10 years. This includes the drug discovery phase (2-4 years), where lead compounds are identified through successive structural optimizations to produce lead compounds; the preclinical research phase (1-3 years) for candidate compounds; and the clinical phase (3-7 years). The drug discovery phase is the most fundamental part of small molecule drug development, as screening technologies directly impact the quality of lead compounds, R&D efficiency, costs, and the likelihood of success. AI is already integrated into the entire drug development pipeline, with broad applications in target discovery, protein structure prediction, virtual compound screening, ADMET prediction, and clinical trial design, including patient recruitment. AI can also assist in predicting clinical trial outcomes, helping to significantly reduce R&D expenses.
Generative AI and ChatGPT
Generative AI, a branch of artificial intelligence, focuses on learning patterns from existing data and generating content similar to the original training data. Unlike rule-based and template-driven AI, generative AI demonstrates greater creativity. The emergence of generative AI models like ChatGPT has revealed immense value in both applications and research. In practical use, ChatGPT has shown impressive capabilities in writing papers, reports, and even coding.
ChatGPT is a natural language processing (NLP) model designed specifically for conversational applications. Built on the GPT-3.5 large language model (LLM), ChatGPT leverages supervised fine-tuning (SFT) and reinforcement learning from human feedback (RLHF), where technical experts simulate dialogues to provide learning data for the model. Additionally, ChatGPT is trained with an emphasis on ethical considerations, enabling it to understand and follow user instructions and generate human-preferred responses.
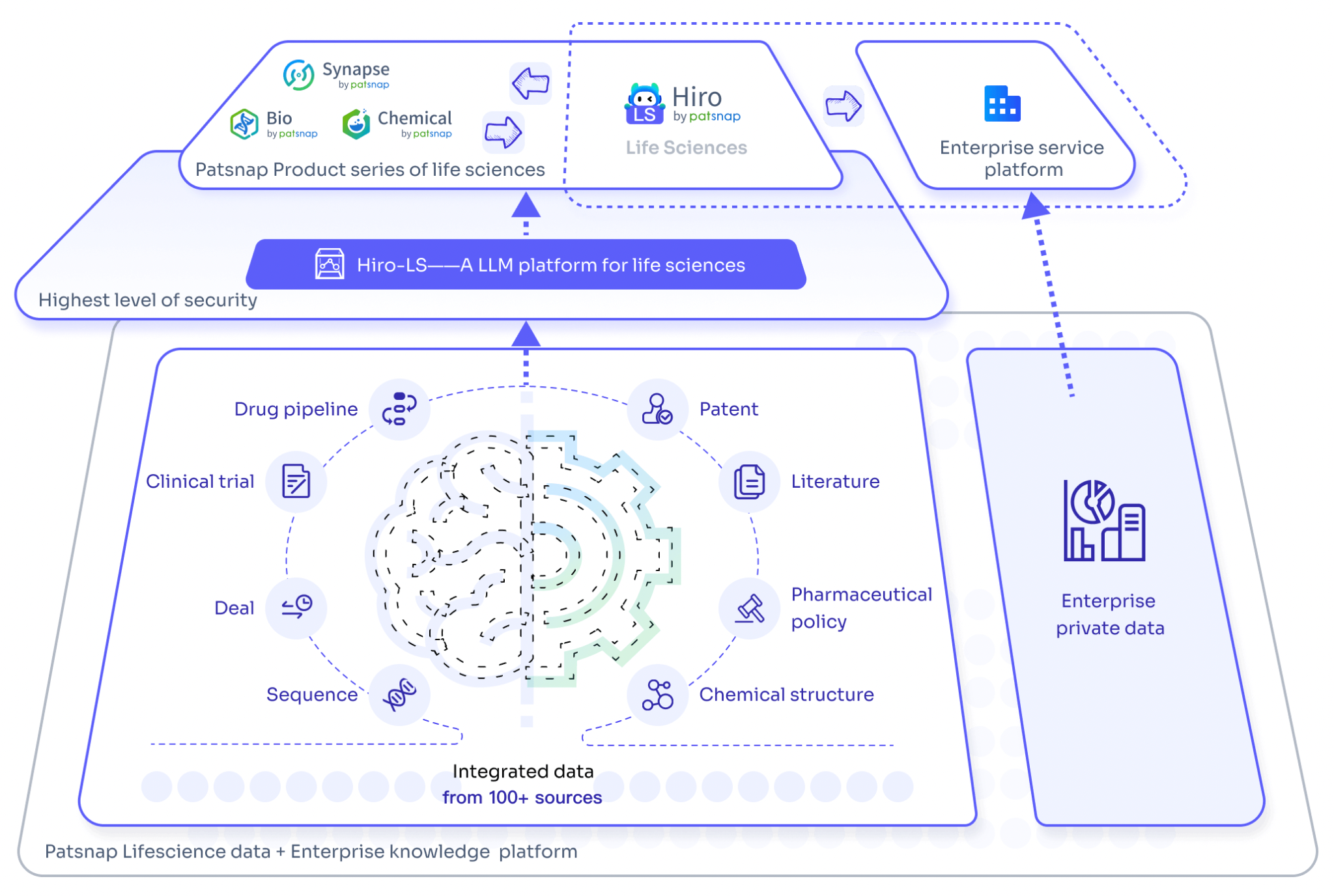
PatSnap's AI Model Hiro-LS for Biopharmaceuticals
Hiro-LS, PatSnap's biopharmaceutical AI model, integrates the proprietary PharmaGPT, a large-scale AI model with 70 billion parameters. PharmaGPT combines knowledge augmentation and industry logic to ensure accurate and compliant information processing, effectively addressing the issue of "hallucination" in AI. By incorporating advanced algorithms such as named entity recognition (NER), chemical structure image recognition, and pharmaceutical knowledge graphs, PharmaGPT establishes a comprehensive data processing framework. During model training, over 200 A800 GPUs were used to process approximately 200 GB of raw text data, integrating more than 16 million pharmaceutical patents, 10 million life science papers, 1 million industry news reports, 200,000 clinical trial datasets, and 50,000 pharmaceutical policy documents. This deep vertical data training ensures that Hiro-LS can provide users with a one-stop, highly reliable data query and response platform.
PharmaGPT has demonstrated superior performance compared to GPT-4 across multiple dimensions, including exam capabilities, machine translation, and classification skills. When users input queries related to pharmaceutical competition landscapes, clinical trials, evidence-based medicine, or sequence/compound patents, Hiro-LS delivers answers that outperform GPT-4. Hiro-LS transforms the question through a system conversion, effectively searching PatSnap's database, resulting in comprehensive responses to pharmaceutical queries, leveraging PatSnap's vast database matrix, enriched by AI models and human-curated API data.
For an experience with the large-scale biopharmaceutical model Hiro-LS, please click here for a quick and free trial of its features!
AI Core Patent Recommendation in Synapse Database
In the early stages of drug development, it is often necessary to focus on information about positive drugs, including code names, structures, clinical information, and core patents. For positive drugs with disclosed structures, compound patents can be easily retrieved through structural searches to gather intelligence. However, some drugs have not yet disclosed their structural information, revealing only molecular codes. In such cases, researchers need to infer the core patents and possibly deduce the molecular structure based on various information sources.
By inputting the code of an undisclosed molecule into Synapse Database, the AI model can analyze relevant patent information (such as the patent applicant, filing date, priority date, and patent classification) as well as drug details (including registration timelines, clinical trials, drug targets, indications, and original company). The model automatically recommends the most likely core patents, with an accuracy rate of up to 95%.
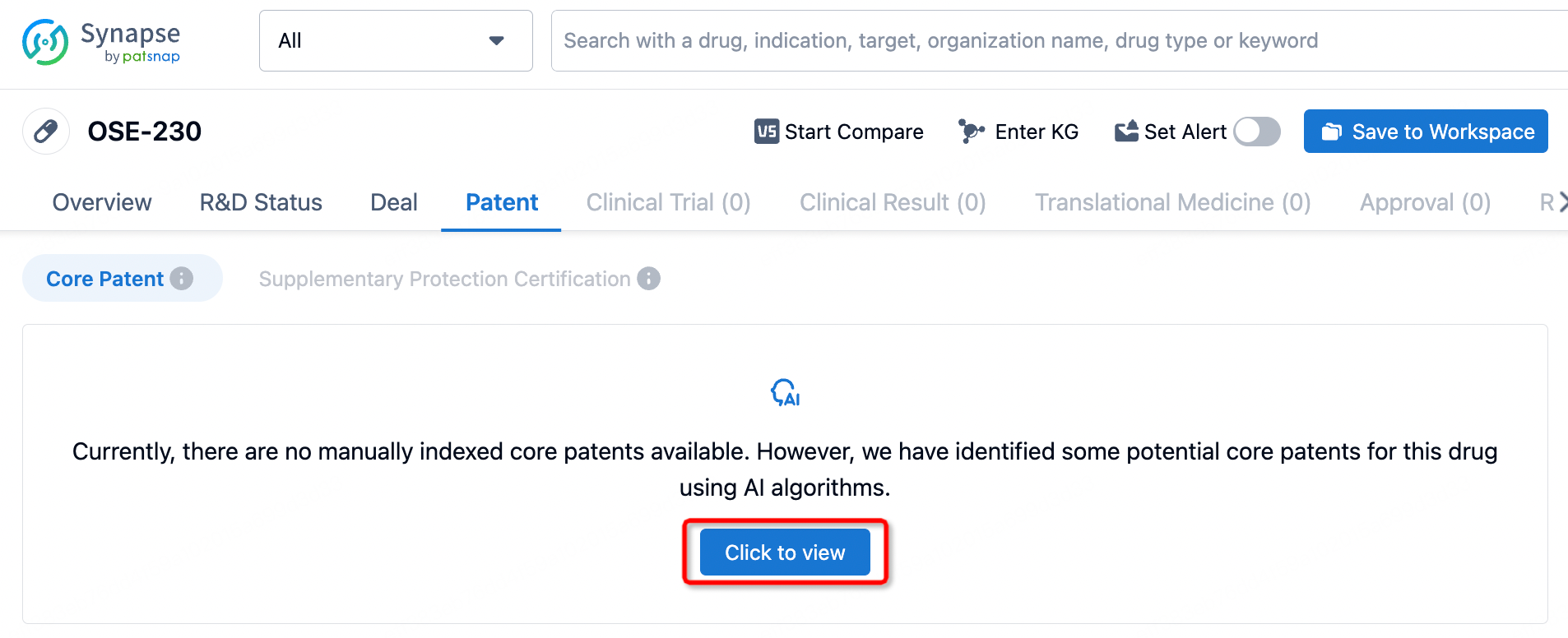
On February 28, 2024, AbbVie and OSE Immunotherapeutics announced a strategic partnership to develop a potential "first-in-class" monoclonal antibody, OSE-230, aimed at addressing chronic and severe inflammation. Under the agreement, OSE Immunotherapeutics could receive over $700 million in upfront and milestone payments. Since the structure of the OSE-230 molecule has not been disclosed, Synapse Database AI model can infer the antibody's core sequence patents.
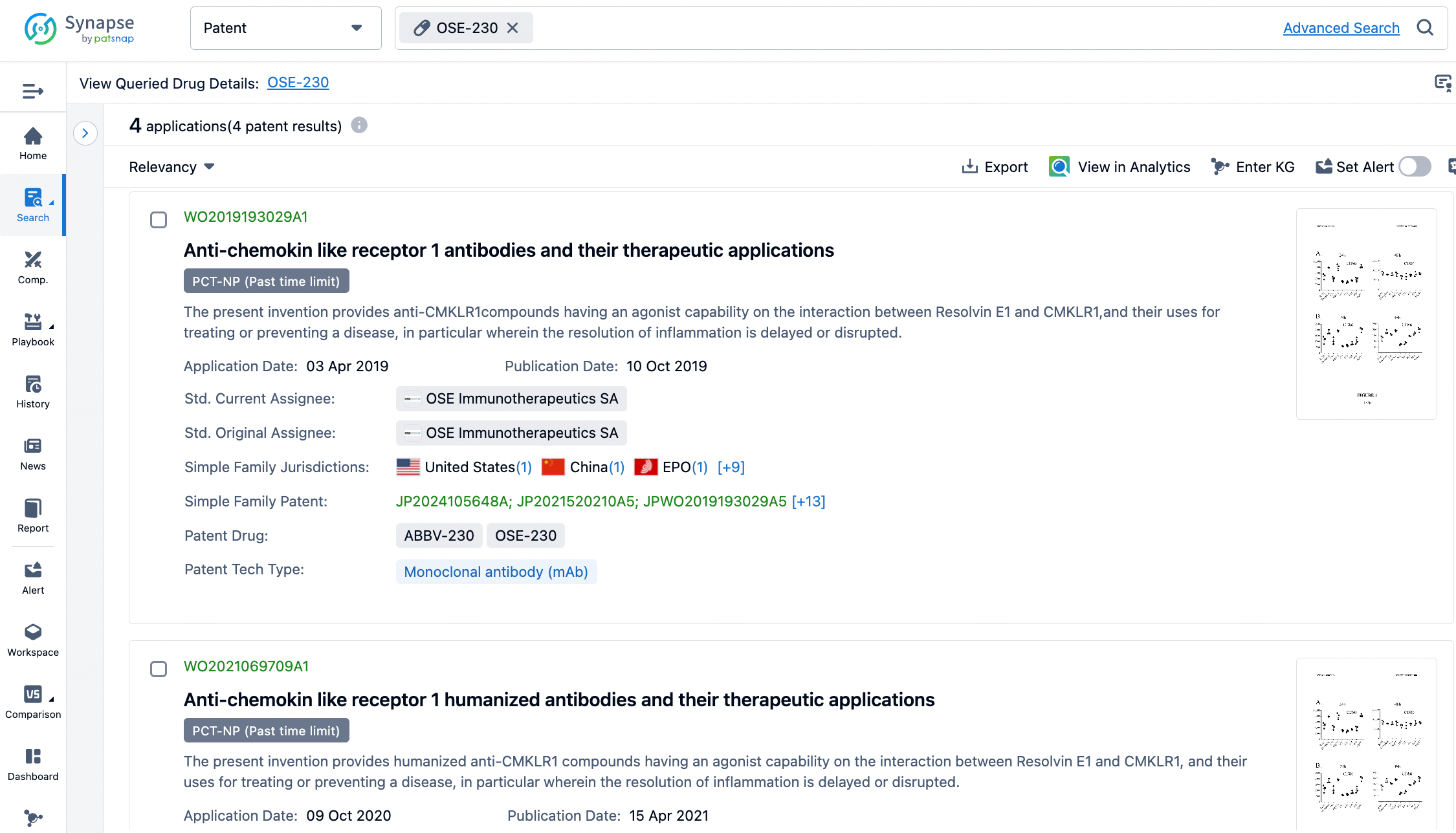
When entering OSE-230 into the Synapse Database, no sequence structure for the antibody is found. Clicking on the "Patent" button shows a message: "Currently there are no manually indexed core patents available. However, we have identified some potential core patents for this drug using AI algorithms." Upon reviewing, four patent groups were identified, including core sequence patents for OSE-230 (patent numbers WO2019193029A1, WO2021069709A1, WO2024028509A2, and WO2024028508A2). Researchers can then easily combine this data with other publicly available information or SAR data to identify potential positive antibodies.
On March 12, 2024, Boehringer Ingelheim and Sosei Heptares announced a global collaboration and exclusive option agreement. The focus of the agreement is to jointly develop and commercialize Sosei Heptares' potential "first-in-class" GPR52 agonist candidate therapies. GPR52 is a G-protein-coupled receptor (GPCR) that could improve outcomes for patients with schizophrenia by addressing positive, negative, and cognitive symptoms. Sosei Heptares may receive over €700 million in upfront and milestone payments.
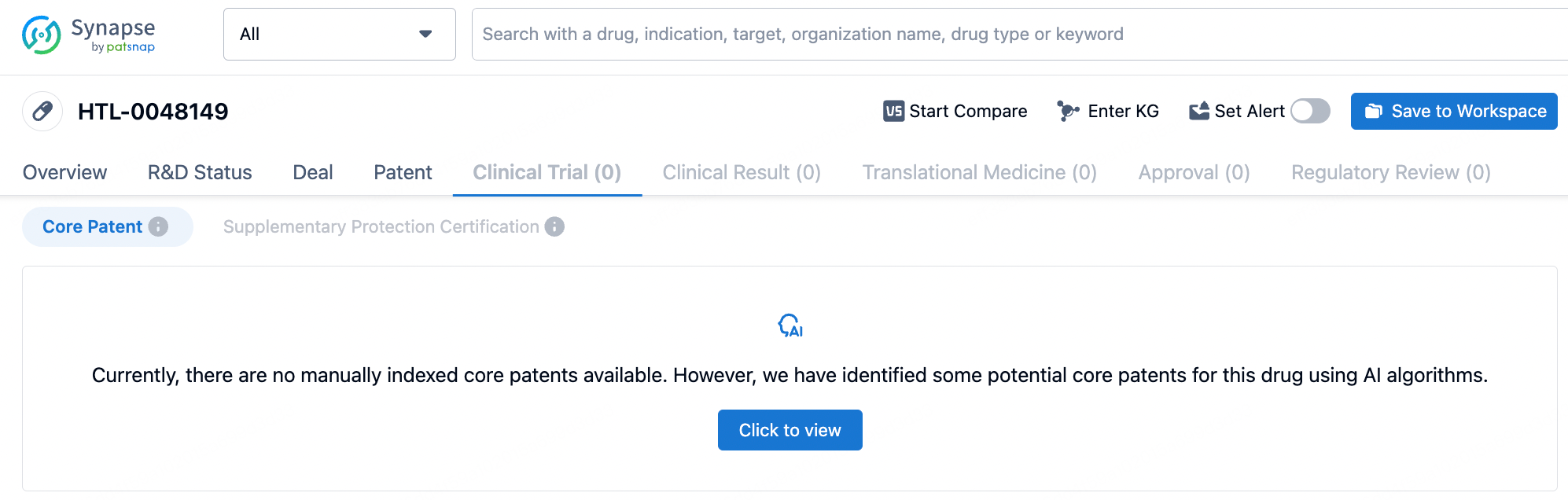
Under the agreement, Boehringer Ingelheim has the option to acquire the development rights for Sosei Heptares' potential "first-in-class" GPR52 agonist, HTL0048149. The licensed candidates will include HTL0048149 as well as several differentiated backup compounds designed by Sosei Heptares using its StaR technology and structure-based drug design (SBDD) platform. Since the structure of the small molecule HTL0048149 has not been disclosed, Synapse Database AI model can infer the relevant core compound patents for this molecule.
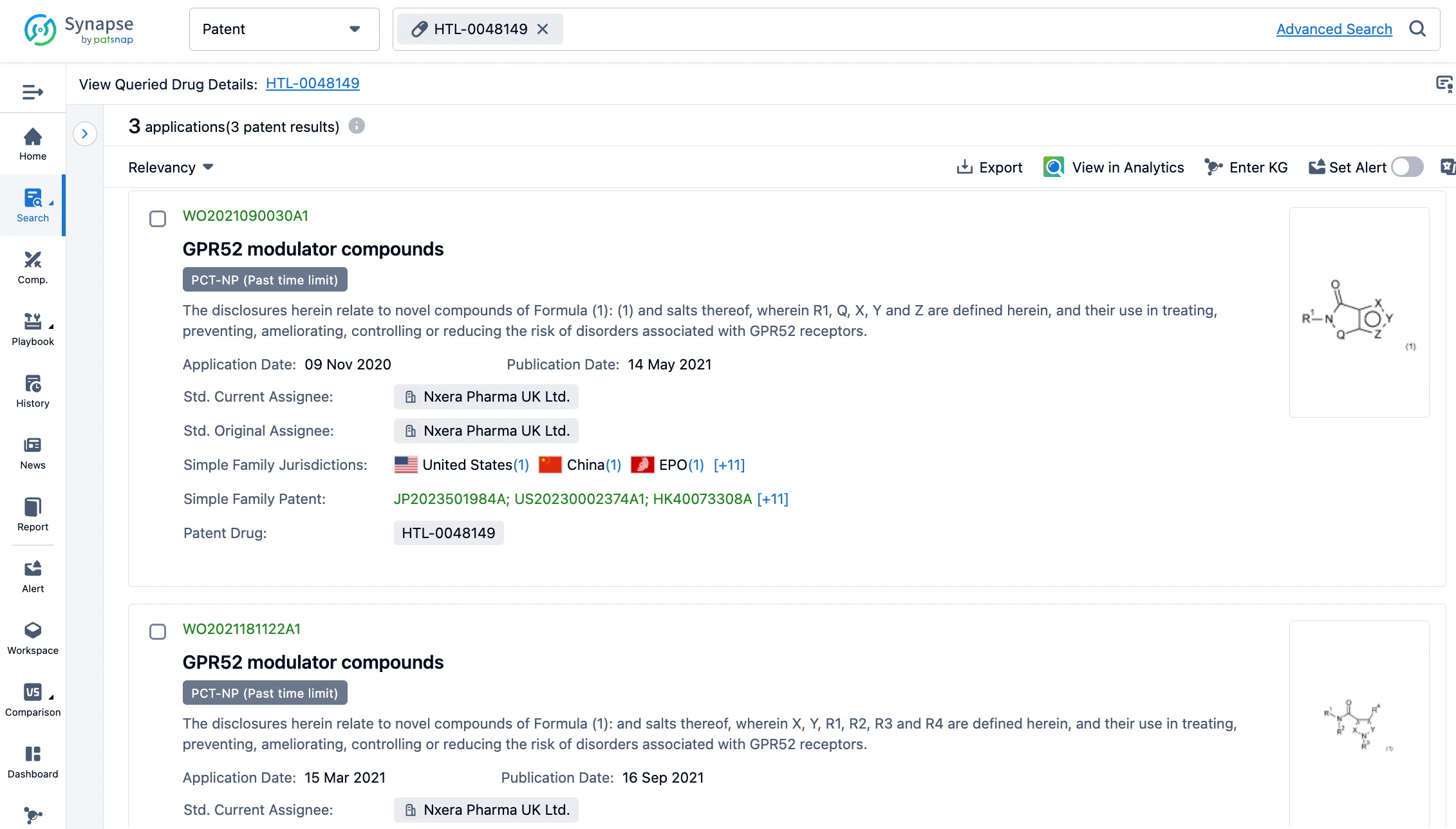
By entering HTL0048149 into the Synapse Database, no disclosed structure for the small molecule was found. Upon clicking the "Patent" button, a message appears: "Currently there are no manually indexed core patents available. However, we have identified some potential core patents for this drug using AI algorithms." Upon reviewing, three groups of patents are identified, which include the core compound patents for HTL0048149 (patent numbers WO2021090030A1, WO2021181122A1, WO2022043714A1). Researchers can conveniently cross-reference the general formulas in the patents and analyze the biological evaluation data provided in the examples to determine the possible structure of the positive compound.
Whether it is large molecule antibodies, ADCs, siRNA, or small molecule clinical candidate compounds, by searching the code in PatSnap's AI core patent recommendation feature, researchers can find the most likely core patents, significantly improving work efficiency.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!