Akari Therapeutics Merges with Peak Bio to Expand ADC Portfolio
Akari Therapeutics, Plc, alongside Peak Bio Inc., has come to a conclusive accord to unite their businesses on equal terms through a stock-for-stock deal. Upon completion of the merger, the integrated company will retain the name Akari Therapeutics, Plc, and is anticipated to maintain its current listing status on the Nasdaq Capital Market under the ticker symbol AKTX.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Upon completion of the transaction, the entity will possess a diversified product portfolio that includes a range of promising compounds at various stages of research and development. There are plans to conduct a strategic evaluation of this portfolio, which will consist of setting priorities for different projects, updating development schedules, identifying potential short-term revenue-generating initiatives, and factoring in additional strategic elements. Some salient features of the integration involve:
The combined product portfolio showcases a powerful Antibody-Drug Conjugate (ADC) platform equipped with innovative payloads and linker mechanisms. The new entity intends to forge state-of-the-art therapeutic options for those suffering from cancer by integrating chemotherapeutic approaches with immunotherapeutic principles. Furthermore, the development slate includes an advanced, pre-clinical ADC that targets TROP-2.
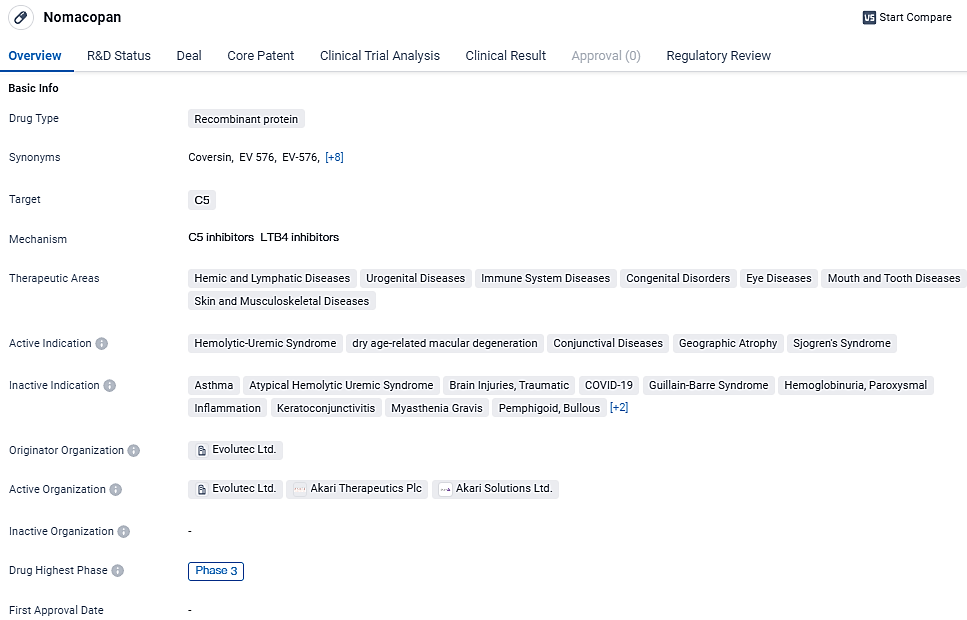
Within the portfolio, Akari’s drug nomacopan, which acts as a dual inhibitor of the complement component C5 and leukotriene B4, is currently under investigation in a Phase 3 trial focusing on the pediatric demographic with thrombotic microangiopathy post-hematopoietic stem cell transplant (HSCT-TMA). This treatment could emerge as the inaugural therapy for HSCT-TMA, a rare but deadly post-transplant adversary, with a notably high mortality rate in severe cases among both adults and children.
An enhanced iteration of nomacopan, with a prolonged duration of activity known as PASylated-nomacopan, is nearing the end of its pre-clinical phase with the aim to tackle geographic atrophy (GA). Its potential involves fulfilling critical patient needs by extending the intervals between administrations of intravitreal injections and mitigating the hazard of choroidal neovascularization—a risk inherent in existing, approved complement inhibitor treatments for GA.
Peak Bio is advancing its Phase 2-ready program, PHP-303, which zeroes in on alpha-1 antitrypsin deficiency (AATD). Acquired under a license from Bayer Healthcare, PHP-303 represents a fifth-generation inhibitor of neutrophil elastase, focusing specifically on the inflammatory dimension of AATD, which is a scarcely diagnosed condition.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
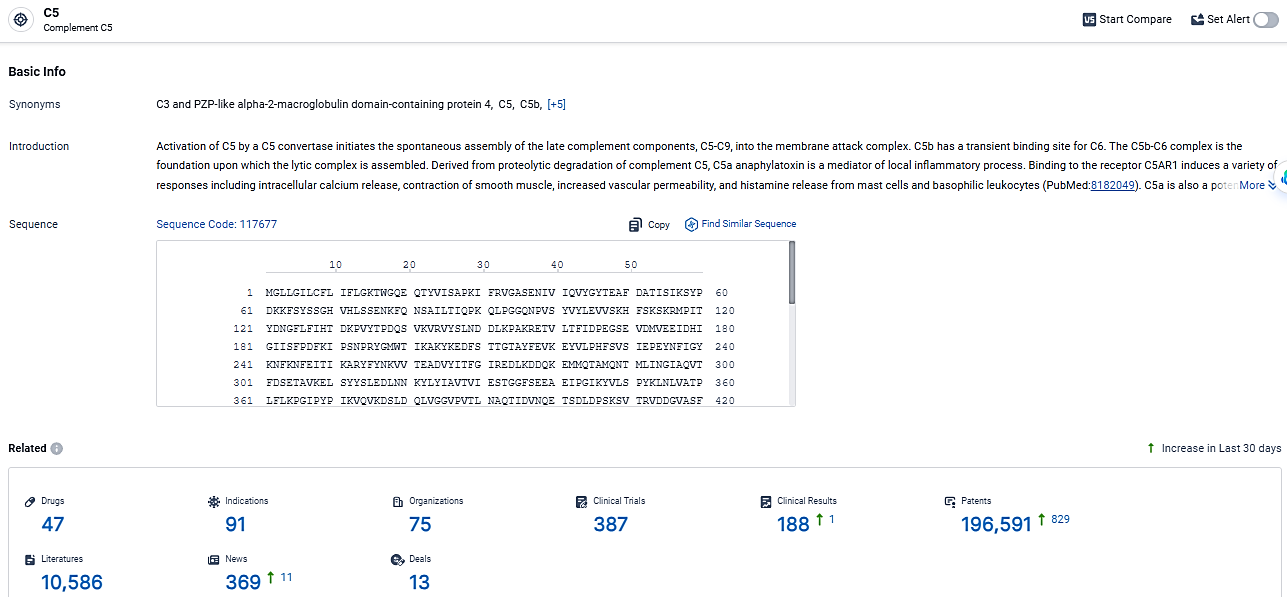
According to the data provided by the Synapse Database, As of March 7, 2024, there are 47 investigational drugs for the C5 target, including 91 indications,75 R&D institutions involved, with related clinical trials reaching 387, and as many as 196591 patents.
Nomacopa targets the C5 protein and is currently in Phase 3 of clinical development. It is being investigated for its potential in treating indications such as hemolytic-uremic syndrome, dry age-related macular degeneration, conjunctival diseases, geographic atrophy, and Sjogren's syndrome. Nomacopan has received regulatory designations such as Rare Pediatric Disease, Fast Track, and Orphan Drug, highlighting its potential to address unmet medical needs in rare diseases.