Almirall Secures Exclusive License for ZKN-013 from Eloxx for Rare Skin Disease Treatment
Almirall, S.A. alongside Eloxx Pharmaceuticals, Inc. have publicly disclosed the formation of a unique licensing partnership concerning the compound known as ZKN-013. As per the terms of this partnership, Almirall has secured the exclusive global privileges to both advance and market ZKN-013, covering its application in rare skin-related medical conditions.
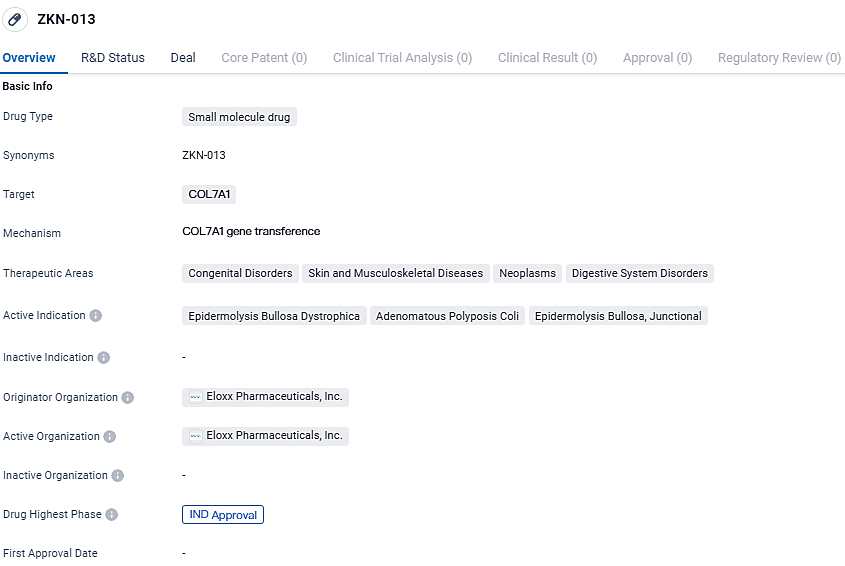
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
ZKN-013 is emerging as a viable candidate as an oral therapeutic agent for readthrough of nonsense mutations, facilitating the generation of effective proteins within host cells. This process directly addresses the foundational issues causing certain infrequent dermatologic conditions and possibly other illnesses.
Under the terms of the deal, Eloxx will be granted an initial payment of $3 million from Almirall, with the prospect of further financial disbursements dependent on progress in the development stages, including both regulatory and commercial benchmarks that could reach a total of $470 million. Moreover, the agreement entails progressive royalties contingent upon any future international sales.
Sumit Aggarwal, Eloxx's President and CEO, expressed enthusiasm about the partnership with Almirall to advance and market ZKN-013, the flagship compound derived from their TURBO-ZM™ platform. He anticipates this compound could substantially influence the management of these severe and distressing disorders.
Karl Ziegelbauer, EVP of R&D and CSO at Almirall, concurred that this licensing deal corresponds with the company's objective to innovate therapeutic solutions for individuals with skin conditions, inclusive of uncommon pathologies. He anticipates the continued development of ZKN-013 will lead to a meaningful therapeutic option for individuals afflicted by critical ailments stemming from nonsense mutations.
Rare dermatological disorders like RDEB/JEB are marked by anomalies in the Collagen7 gene, which plays a crucial role in the formation and protective capabilities of the skin. ZKN-013 has shown compelling efficacy in functional preclinical evaluations using cells from RDEB/JEB patients and APCmin mouse models. These tests showed the agent's ability to incite the production of intact and functional COL7 protein in cells derived from patients with RDEB.
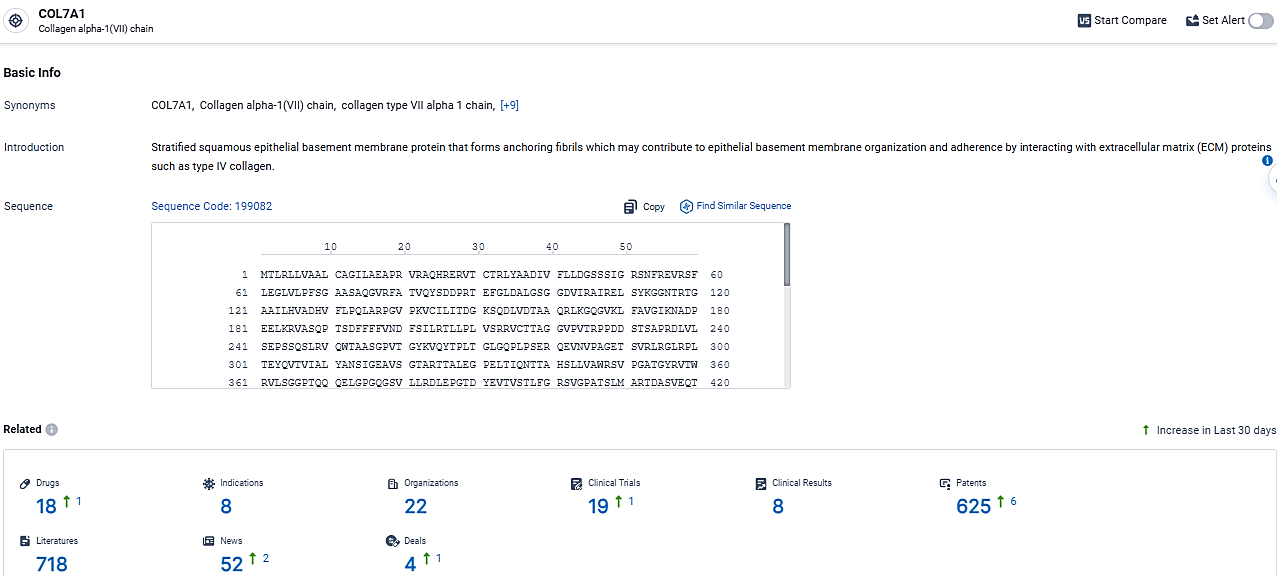
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 17, 2024, there are 18 investigational drugs for the COL7A1 target, including 8 indications, 22 R&D institutions involved, with related clinical trials reaching 19, and as many as 625 patents.
ZKN-013 is also being developed for the treatment of FAP patients with nonsense mutations characterized by proliferation of colon polyps and progression to colon cancer. ZKN-013 has reached the highest phase of development, with IND approval obtained, allowing for clinical trials to be conducted. Further research and regulatory processes will be required to determine the full potential of ZKN-013 in addressing these congenital disorders, skin and musculoskeletal diseases, neoplasms, and digestive system disorders.