An analysis of ASKC202's R&D progress and its clinical results presented at the 2024 AACR Annual Meeting
On April 5, 2024, the AACR reported the latest clinical trial results of ASKC202, illustrating its potential clinical benefits and setting the stage for further exploration.
ASKC202's R&D Progress
ASKC202 is a small molecule drug that targets c-Met, a protein involved in cell growth and survival. It is being developed by Jiangsu Aosaikang Pharmaceutical Co. Ltd. The drug is primarily focused on treating neoplasms and respiratory diseases.
According to the Patsnap Synapse, ASKC202 is still in the early stages of development. And the clinical trial distribution for ASKC202 is primarily in China. The key indication is Advanced Malignant Solid Neoplasm.
Detailed Clinical Result of ASKC202
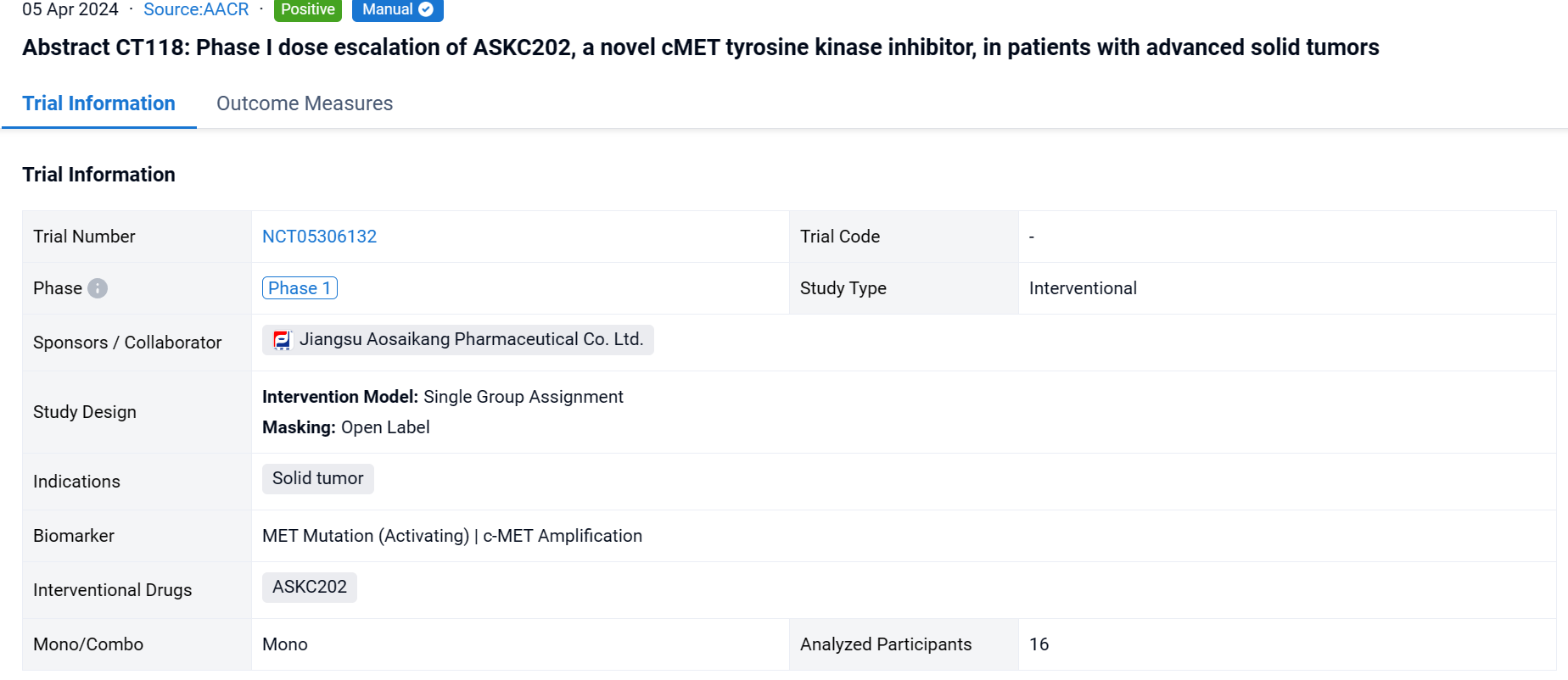
This single group assignment, open-labeled clinical study (NCT05306132) was aimed to evaluate preliminary safety and efficacy of ASKC202, a novel cMET tyrosine kinase inhibitor, in patients with advanced solid tumors.
In this study, a two-part study was initiated to evaluate MTD, safety and tolerability, pharmacokinetics and preliminary antitumor activity of ASKC202, including dose escalation study (Part A) and dose expansion study (Part B). Responses were assessed per RECIST 1.1 every 2 cycles (6 weeks). AEs were graded using CTCAE v5.0.In Part A, dose escalation followed the accelerated titration combined with the "3+3" design. Patients with heavily pre-treated solid tumors received ASKC202 orally at doses of 50, 150, 300, 450 and 600 mg/day once daily. The treatment continued until disease progression, unacceptable toxicity or treatment termination. If the subject in the 50mg dose group developed DLT, the dose group was switched to a "3+3" design.
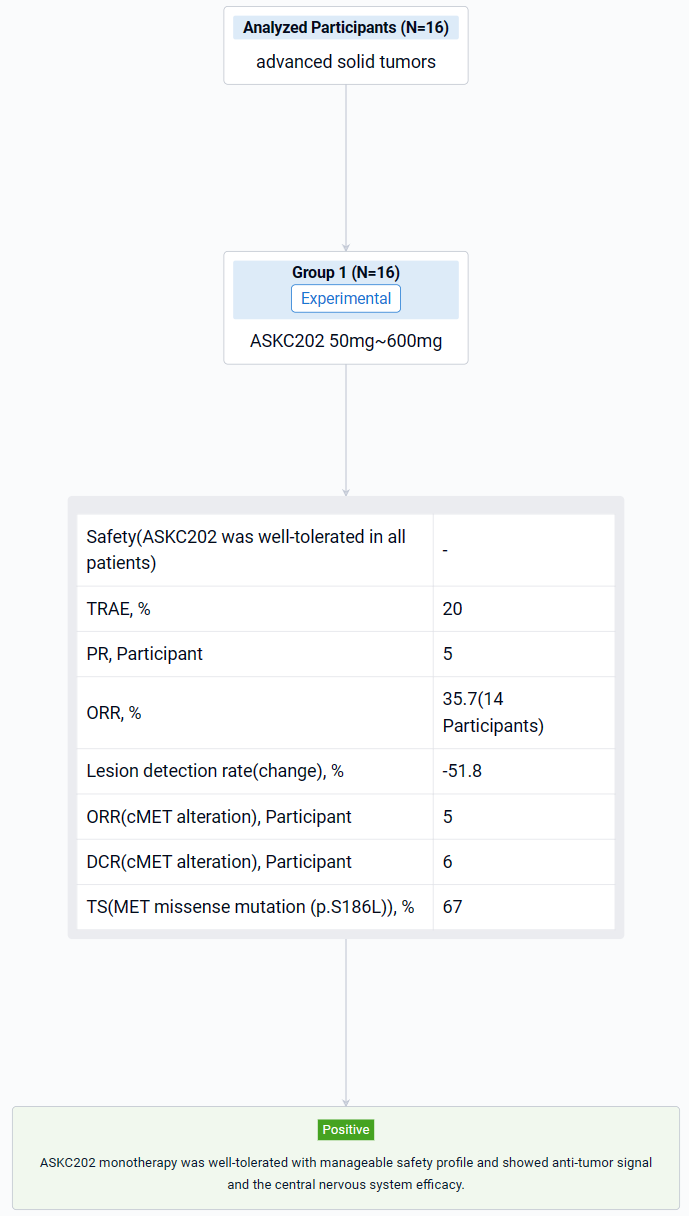
The result showed that as of November 30, 2023, 16 patients who are refractory or don’t respond to standard therapy were available received ASKC202 (50mg: N=1; 150mg: N=3; 300mg: N=6; 450mg: N=3; 600mg: N=3). 15/16 pts had pathological confirmation of non-small-cell lung cancer (except for 1 case of PSC). 8/16 pts had brain metastasis. 10/16 pts had cMET dysregulation including MET amplification (N=9; gene copy number 1.3-8.3) and MET missense mutation (N=1; p.S186L) with no patient having Exon 14 skipping mutation.ASKC202 was well-tolerated in all patients. No dose-limiting toxicity was observed in all dose levels (50 to 600 mg). The common TRAEs occurring in more than 20% of patients included: anemia (5/16), blood glucose elevation (5/16), ALT elevation (4/16), AST elevation (4/16) and peripheral edema (4/16). Grade 3 TRAEs were only observed in one patient (300mg QD), including anemia and hypokalemia. Among 14pts who had at least one post-baseline anti-tumor assessment, 5 pts achieved a confirmed partial response. The confirmed ORR is 35.7%. The median best percentage change in target lesion size from baseline (depth of response) was 51.8% and 3pts had duration of tumor response ≥ 6 months. ORR and DCR for patients with cMET alteration (N=8) was 62.5% (5/8) and 75.0% (6/8) respectively. One subject (150mg QD) with MET missense mutation (p.S186L) had a 67% tumor reduction in intracranial lesion from baseline. Exposure of ASKC202 increased dose-proportionally at dose range 50mg to 300mg, but less than dose-proportionally increased at 300mg to 600mg.
It can be concluded that ASKC202 monotherapy was well-tolerated with manageable safety profile and showed anti-tumor signal and the central nervous system efficacy.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
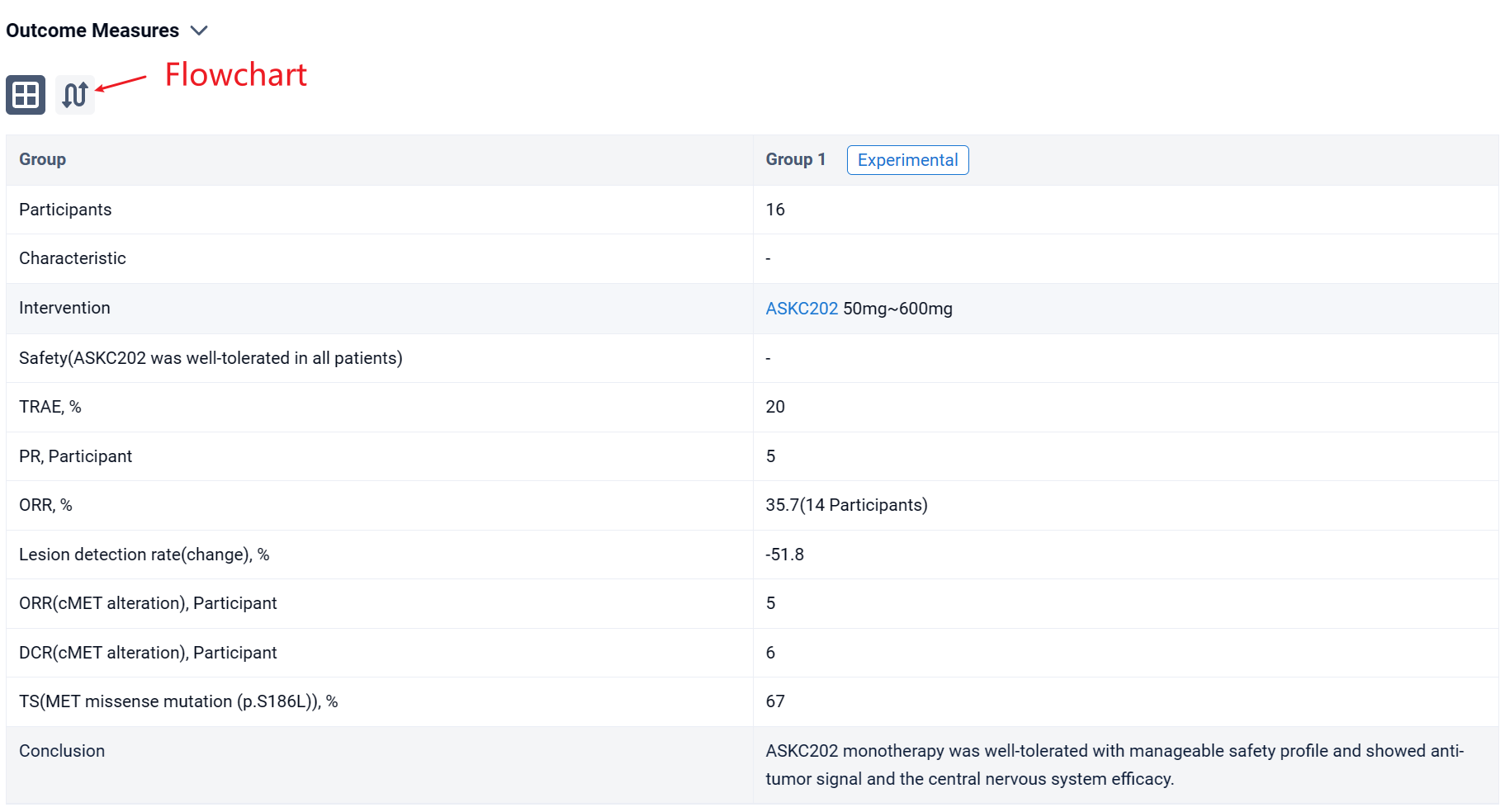

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!