An analysis of Atezolizumab's R&D progress and its clinical results presented at the 2023 AACR Annual Meeting
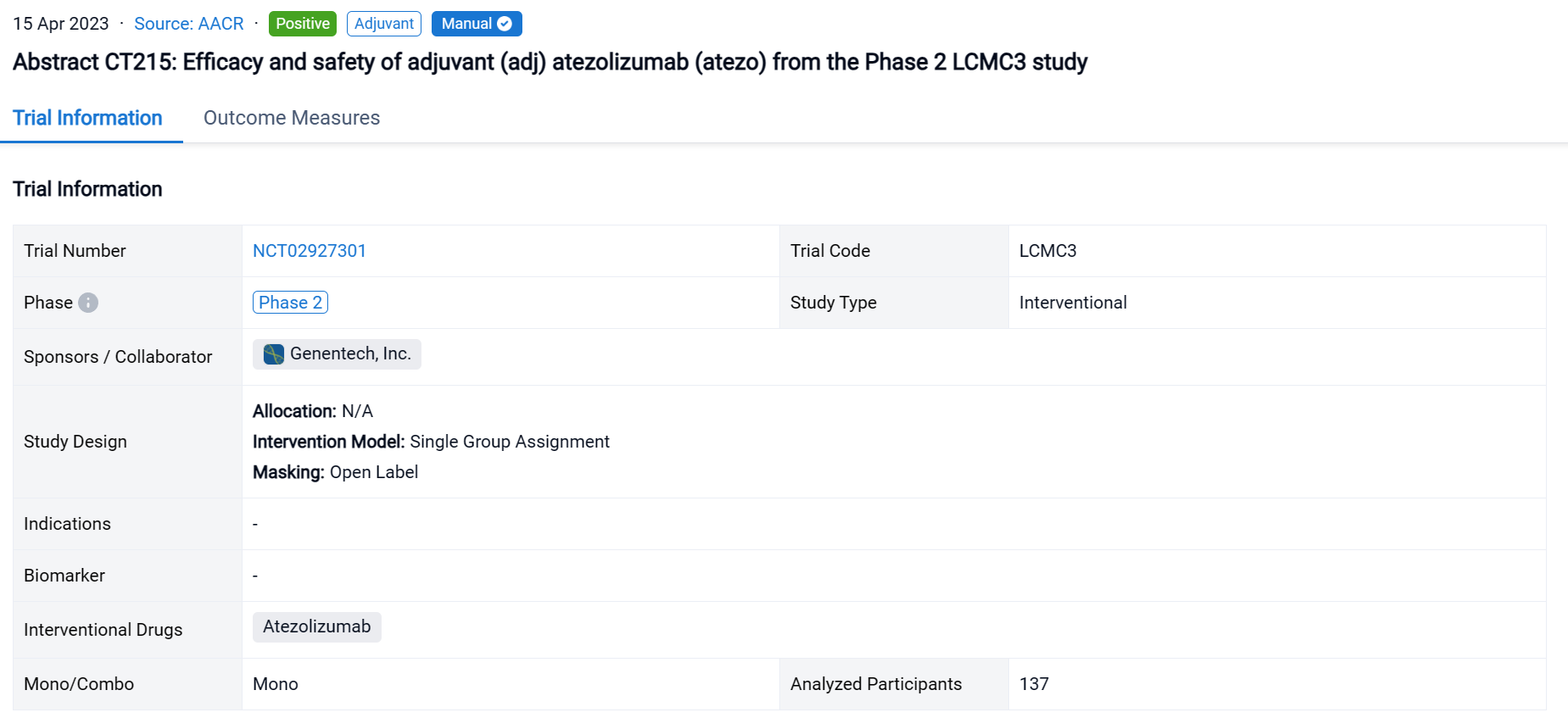
On 15 Apr 2023, the efficacy and safety of adjuvant (adj) atezolizumab (atezo) from the Phase 2 LCMC3 study was reported at the AACR Congress.
Atezolizumab's R&D Progress
Atezolizumab is a monoclonal antibody drug that targets PDL1, a protein involved in regulating the immune system. It has been approved for use in various therapeutic areas, including neoplasms (tumors), hemic and lymphatic diseases, urogenital diseases, nervous system diseases, digestive system disorders, endocrinology and metabolic diseases, immune system diseases, cardiovascular diseases, skin and musculoskeletal diseases, other diseases, and respiratory diseases. 
According to the Patsnap Synapse, Atezolizumab was developed by Universitair Medisch Centrum Groningen and Genentech, Inc. It received its first approval in the United States in April 2017. And the clinical trial areas for Atezolizumab are primarily in the United States, China, and United Kingdom. The key indication is Neoplasms. 
Detailed Clinical Result of Atezolizumab
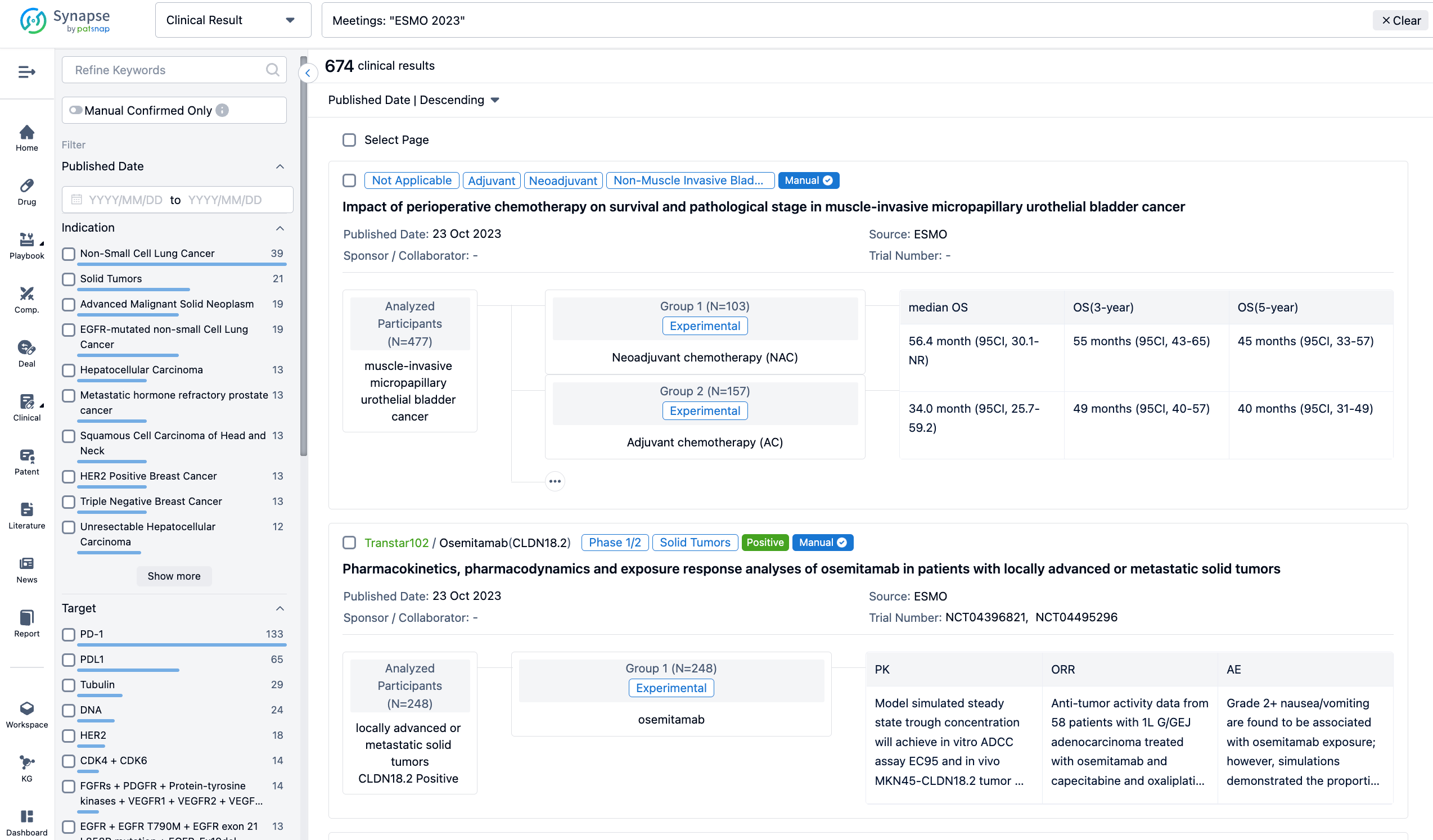
LCMC3 (NCT02927301), an open-label, single-arm Phase 2 study to investigate neoadjuvant (neoadj) and adj atezo (anti-PD-L1) in patients (pts) with early-stage non-small cell lung cancer (NSCLC), met its primary endpoint with a 20% major pathological response (MPR) rate after neoadj atezo. 
In this study, eligible pts aged ≥18 y had resectable stage IB-IIIA or select IIIB NSCLC and ECOG PS of 0 or 1. Pts received neoadj atezo 1200 mg IV for ≤2 cycles (Days 1 and 22) followed by surgery (Day 40±10). Pts deemed to have clinical benefit were offered the option to receive adj atezo every 3 weeks for up to 12 months. The primary endpoint was MPR rate (≤10% viable tumor cells at surgery) in pts without EGFR/ALK mutations. Exploratory endpoints included DFS and OS. Safety was assessed during the adj phase.
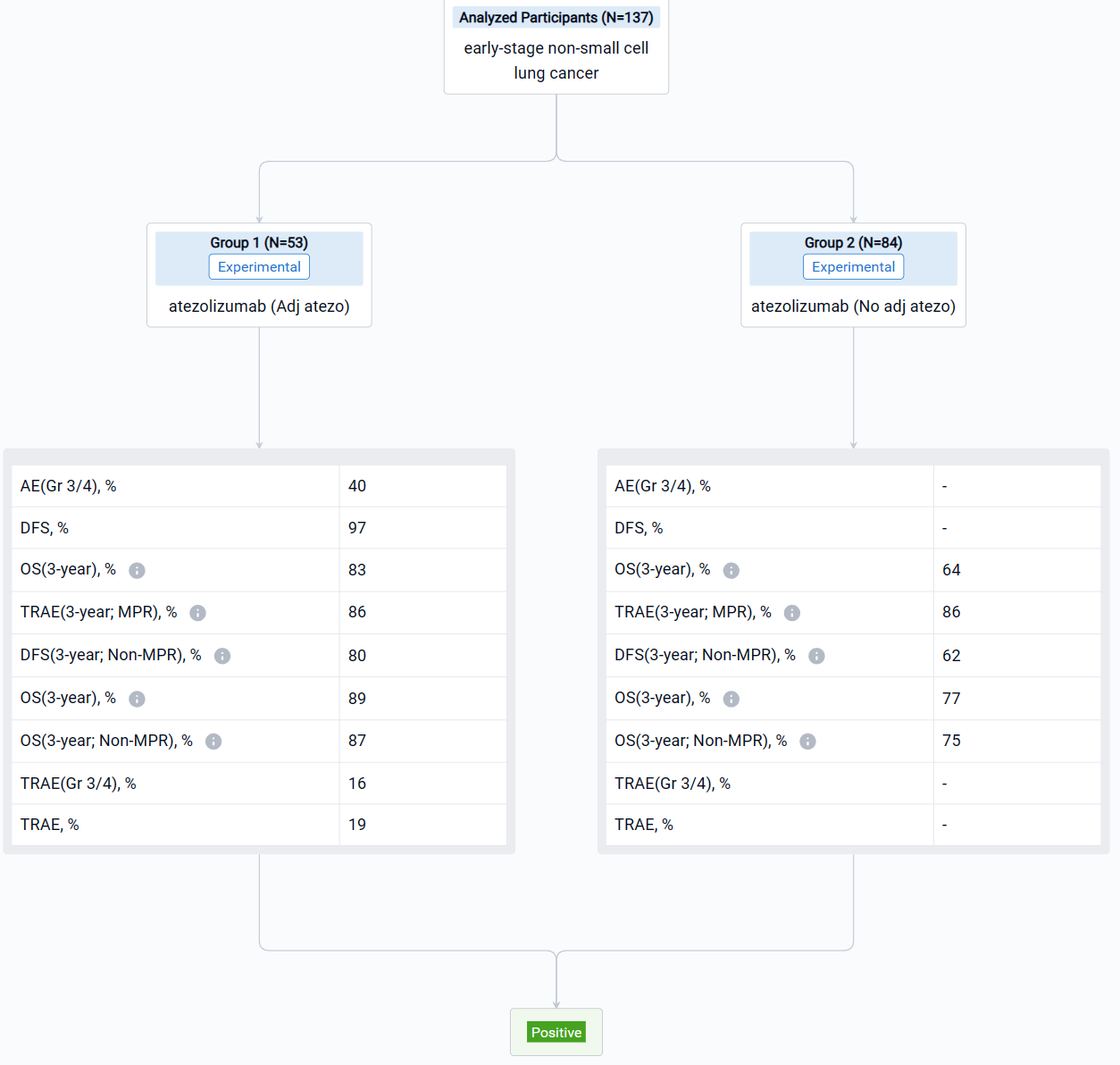
The result showed that Data cutoff was Oct 21, 2022. The primary efficacy population (PEP) was 137 pts without EGFR/ALK alterations who had surgery and MPR assessment. In the PEP, 53 pts (39%) received adj atezo and 84 (61%) did not. The 3-y DFS rate was 83% with adj atezo vs 64% without (HR, 0.43; 95% CI: 0.21, 0.90; P=0.025). 3-y OS and results by MPR status are provided in the table. In the adj atezo safety population (n=57), treatment-related (TR) AEs after adj atezo occurred in 55 pts (97%; Gr 3/4, 40%), including 11 TRAEs (19%; Gr 3/4, 16%) leading to discontinuation of adj atezo. No Gr 5 TRAEs were seen in the adj phase. Multivariate analysis also showed a trend toward better DFS for pts receiving adj atezo vs those with no adj atezo (HR, 0.52; 95% CI: 0.22, 1.21; P=0.126).
It can be concluded that LCMC3 pts with resectable stage IB-IIIA or select IIIB NSCLC who received adj atezo had improved DFS and showed a trend toward improved OS vs pts who did not receive adj atezo. Furthermore, the non-MPR subgroup had the same trend toward improved DFS and OS with adj atezo vs pts who did not receive adj atezo. Adj atezo was well tolerated, with no new safety concerns.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
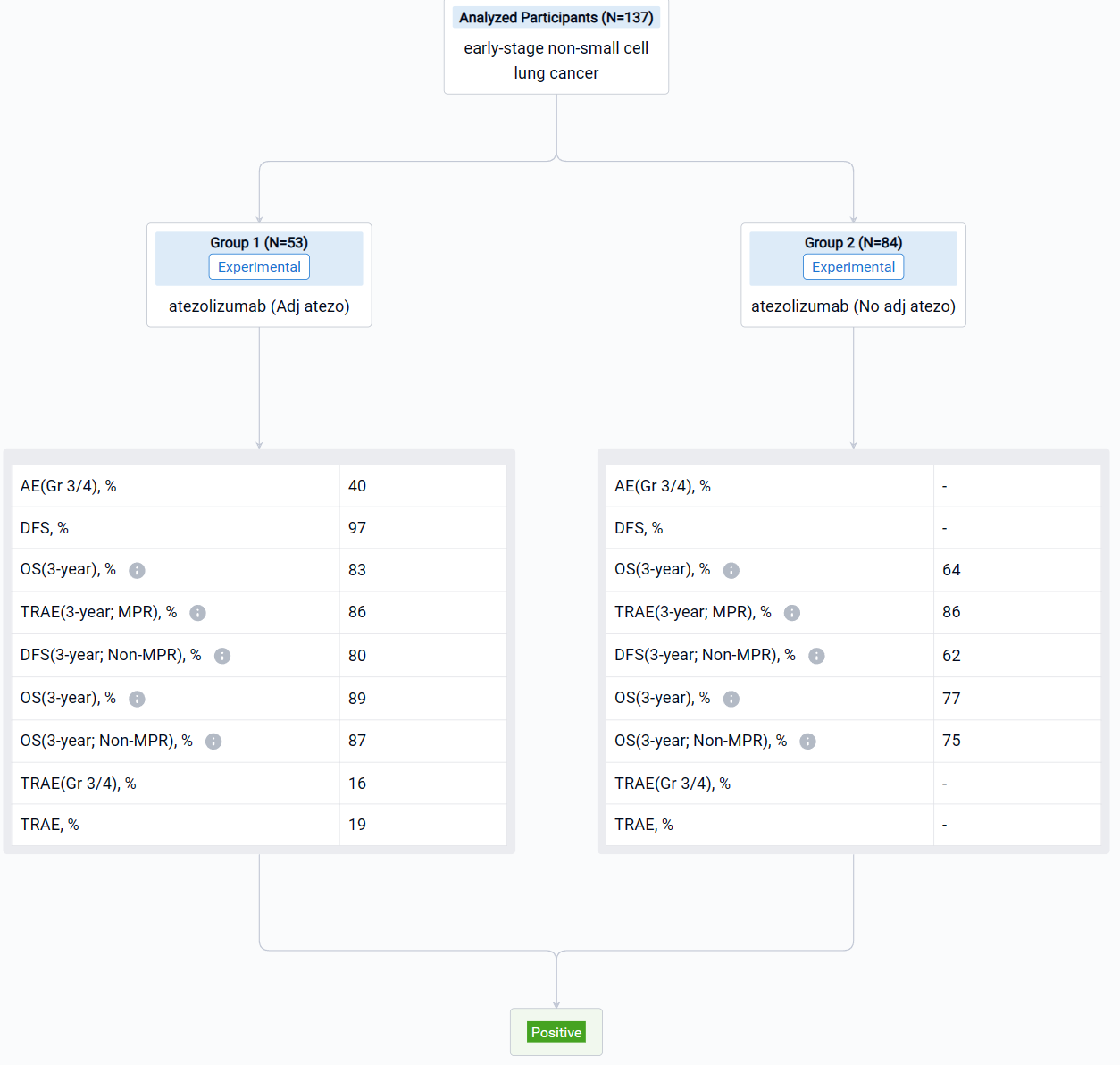
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!