An analysis of UCART-22's R&D progress and its clinical results presented at the 2023 ASH Annual Meeting
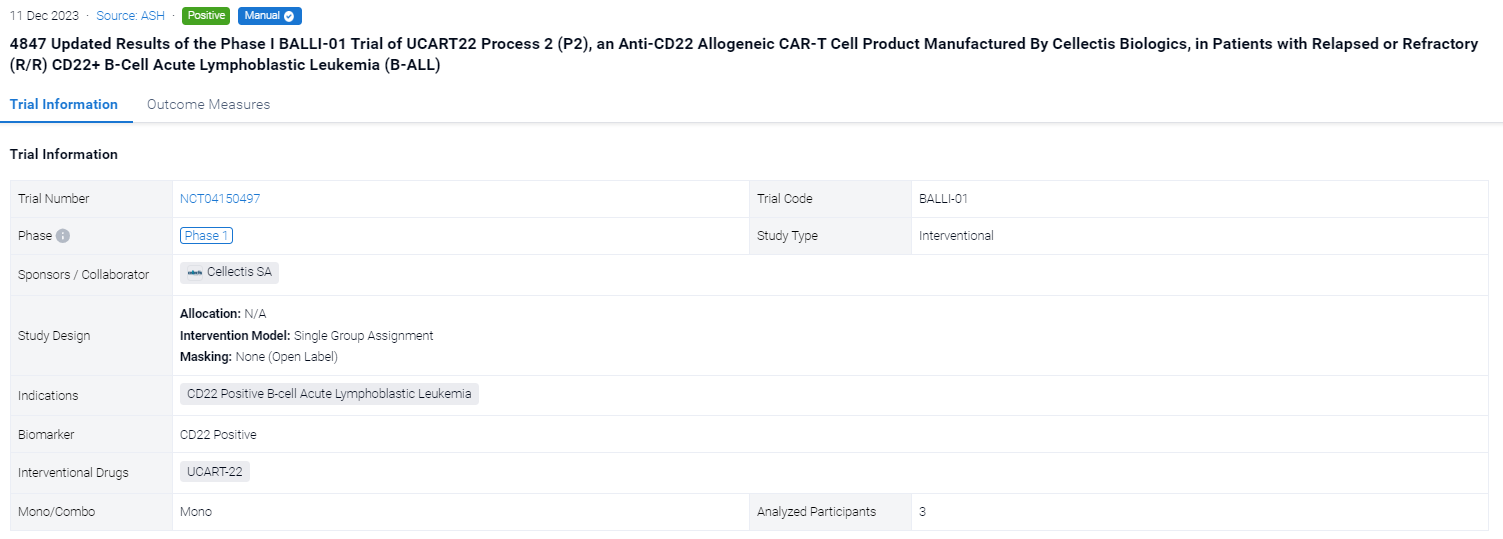
On 11 Dec 2023, the updated Results of the Phase I BALLI-01 Trial of UCART22 Process 2 (P2) in Patients with Relapsed or Refractory (R/R) CD22+ B-Cell Acute Lymphoblastic Leukemia (B-ALL) will be reported in 2023 ASH.
UCART-22's R&D Progress
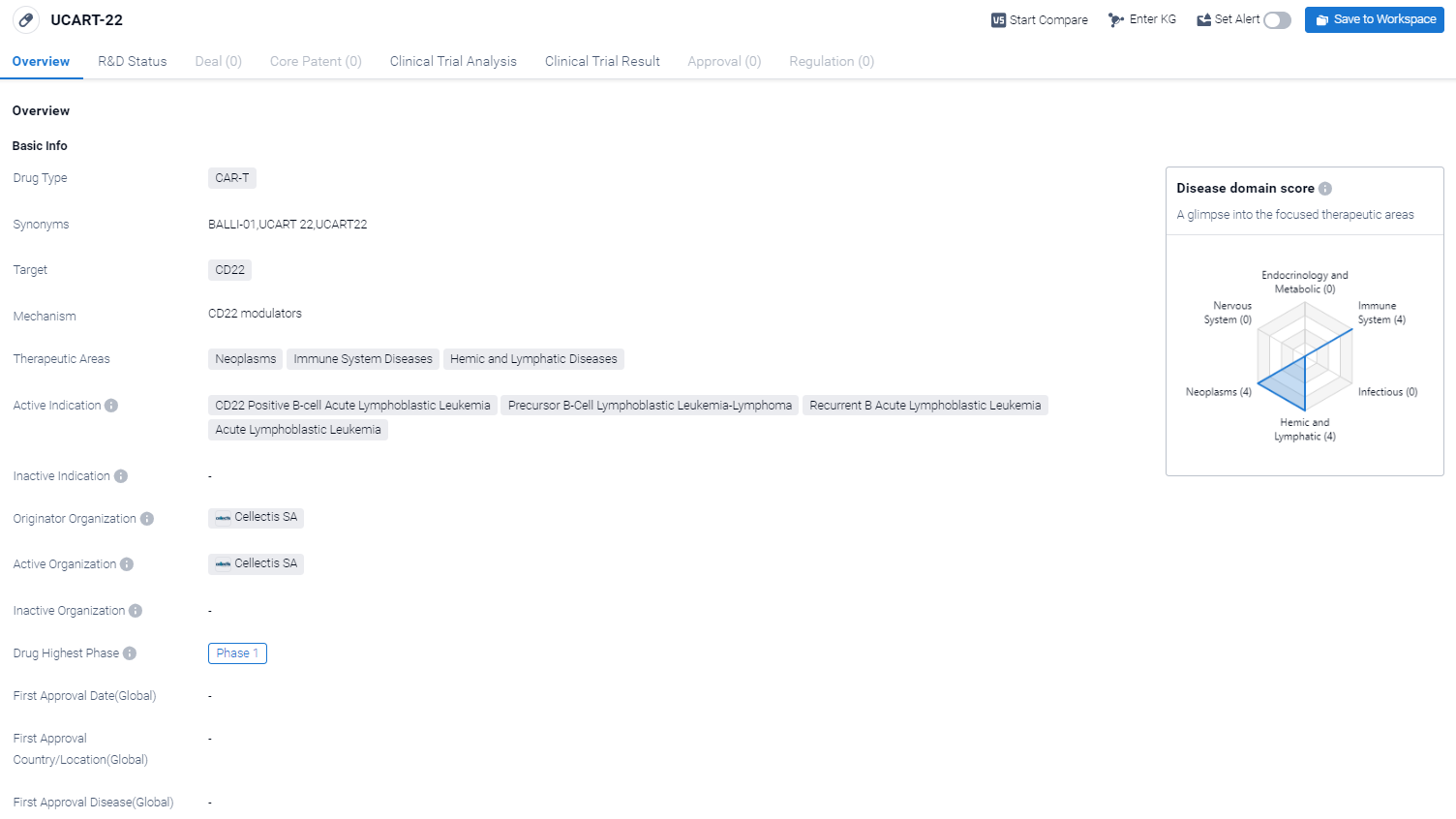
UCART-22 is a CAR-T therapy drug that targets CD22, a protein found on the surface of certain cancer cells. It is being developed by Cellectis SA, a biopharmaceutical company. UCART-22 has shown potential in treating various neoplasms, immune system diseases, and hemic and lymphatic diseases.
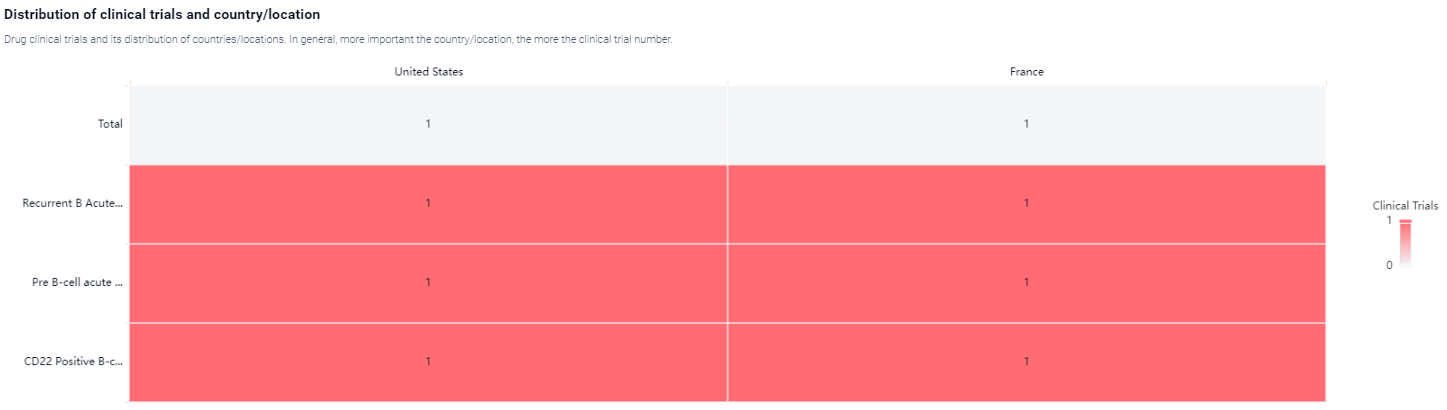
According to the Patsnap Synapse, UCART-22 is currently in Phase 1 of clinical trials. And the clinical trial areas for UCART-22 are primarily in the United States and France. The key indication is Recurrent B Acute Lymphoblastic Leukemia.
Detailed Clinical Result of UCART-22
The single group assignment, open-labeled clinical trial (NCT04150497) was aimed to investigate the safety and preliminary efficacy of UCART-22 in this poor-risk R/R B-ALL population.
In this study, the primary endpoints are safety, tolerability, and determining the MTD/RP2D of UCART22. Additional endpoints are anti-leukemic activity and expansion of UCART22. Eligibility criteria include age 15‒70y, B-ALL blast CD22 expression ≥ 70%, and ≥ 2 prior treatment regimens. After FCA (F 30 mg/m2 × 3d, C 0.5g/m2 × 3d, A 20 mg/d × 3d) LD regimen, pts received a single infusion of UCART22-P2. In vitro comparability assays suggested that UCART22 P2 was more potent than UCART22 P1, so dose escalation with UCART22 P2 started at DL2 (1 x 106 cells/kg) compared to the highest studied dose DL3 (5 x 106 cells/kg) with UCART22 P1.
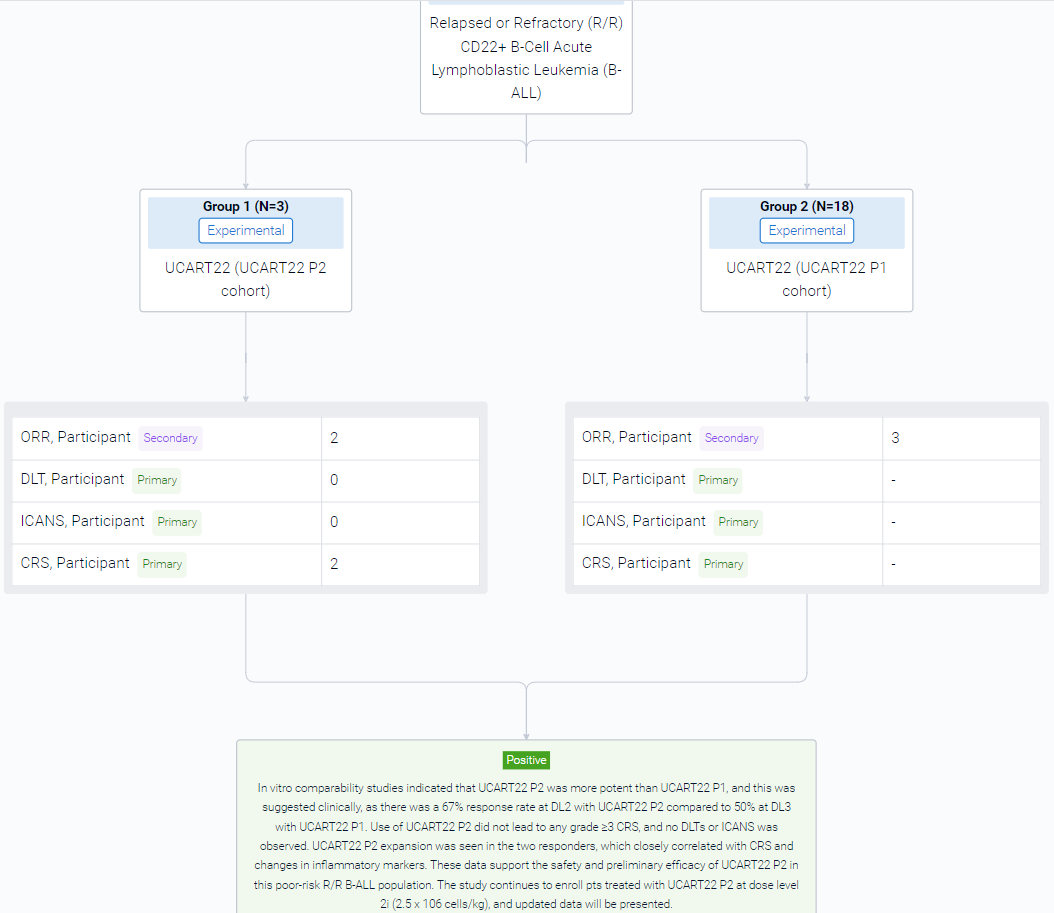
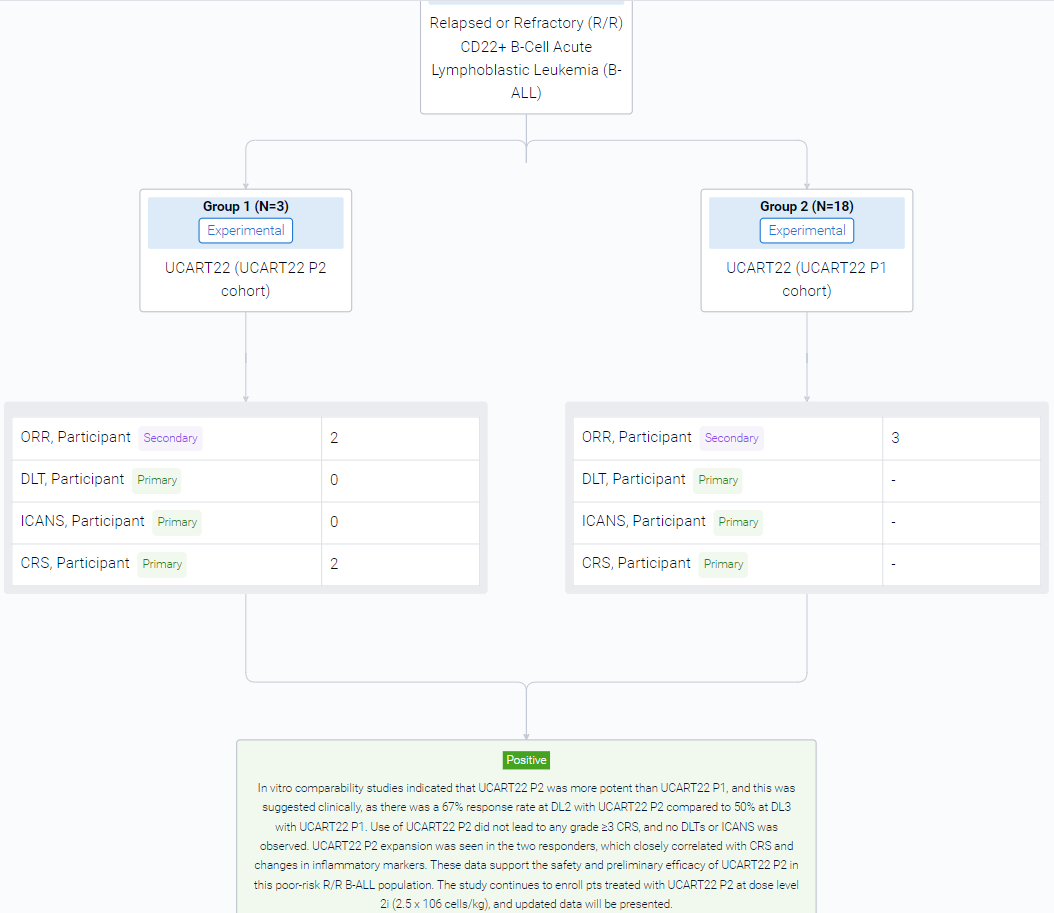
The result showed that as of 01 July 2023, 3 pts were enrolled into the first UCART22 P2 cohort at DL2. Pt 1 is a 17yo female with B-ALL with a hypodiploid karyotype and a germline TP53 mutation whose disease had previously failed to respond to multiagent chemotherapy, blinatumomab (blina), inotuzumab (ino), venetoclax (ven), allogeneic hematopoietic stem cell transplantation (HSCT), and autologous CD19 CAR T-cell therapy (CAR19) x2. Pt 2 is a 68yo female with Ph-negative B-ALL who relapsed with CD19-low disease after multiagent chemotherapy, ino, and blina. Pt 3 is a 27yo male with B-ALL with an ABL2 fusion who had failed multiagent chemotherapy, ino, blina, TKIs, and an experimental autologous CAR19. UCART22 P2 administered after the FCA LD regimen was well tolerated. No DLTs or ICANS were observed. Cytokine release syndrome (CRS) occurred in 2/3 (67%) pts, with one G1 that resolved without treatment and one G2 that resolved after tocilizumab x1. There was one G5 sepsis SAE at D40 considered related to UCART22 P2 and FCA LD in Pt 1. Responses were assessed beginning on D28. Up to FC/FCA-DL3 with 18 pts treated with UCART22 P1, 1 CR, 4 CRi, and 2 morphologic leukemia-free states (MLFS) were observed, with 3 responses occurring out of 6 (50%) patients treated with FCA-DL3 (previously reported, EHA 2023). For UCART22 P2, FCA-DL2, 2/3 pts (67%) responded: Pt 2 achieved an MRD neg CR lasting over 84 days after UCART22 infusion; Pt 1 achieved an MRD negative MLFS up to D40. Pt 3 was refractory to treatment, however this pt received bridging therapy with dasatinib for his ABL2 fusion, and on Day -1, only 47% of the leukemic cells expressed CD22 (down from 88% at screening). UCART22 P2 expansion was observed by flow cytometry in peripheral blood with peak of 79 cells/μl in Pt 1 and 225 cells/μl in Pt 2, both at D11, with predominantly CD8 cells expanding. Inflammatory markers such as ferritin, IFN-γ, TNF-α and IL-6 levels increased more than 3-fold, correlating with UCART22 P2 expansion and CRS.
It can be concluded that in vitro comparability studies indicated that UCART22 P2 was more potent than UCART22 P1, and this was suggested clinically, as there was a 67% response rate at DL2 with UCART22 P2 compared to 50% at DL3 with UCART22 P1. Use of UCART22 P2 did not lead to any grade ≥3 CRS, and no DLTs or ICANS was observed. UCART22 P2 expansion was seen in the two responders, which closely correlated with CRS and changes in inflammatory markers. These data support the safety and preliminary efficacy of UCART22 P2 in this poor-risk R/R B-ALL population. The study continues to enroll pts treated with UCART22 P2 at dose level 2i (2.5 x 106 cells/kg), and updated data will be presented.
How to Easily View the Clinical Results Using Synapse Database?
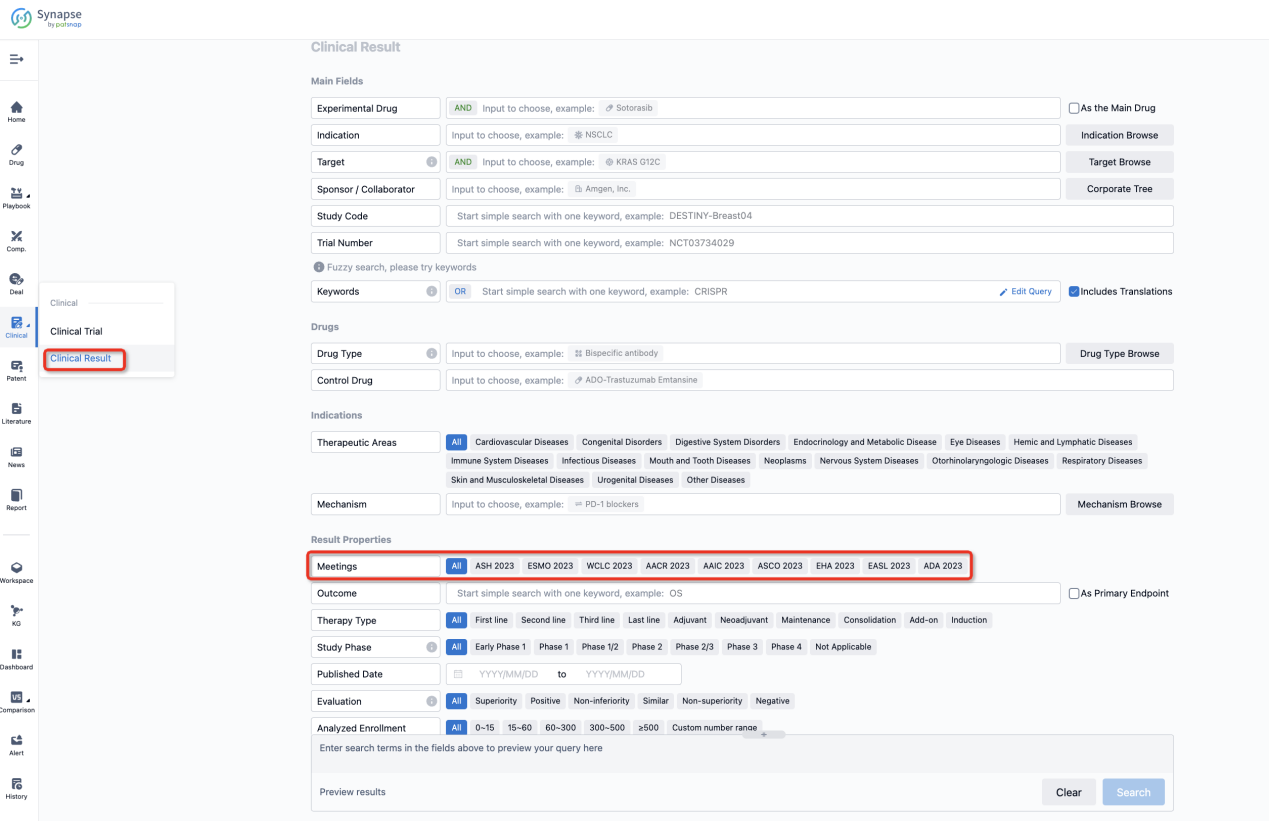
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
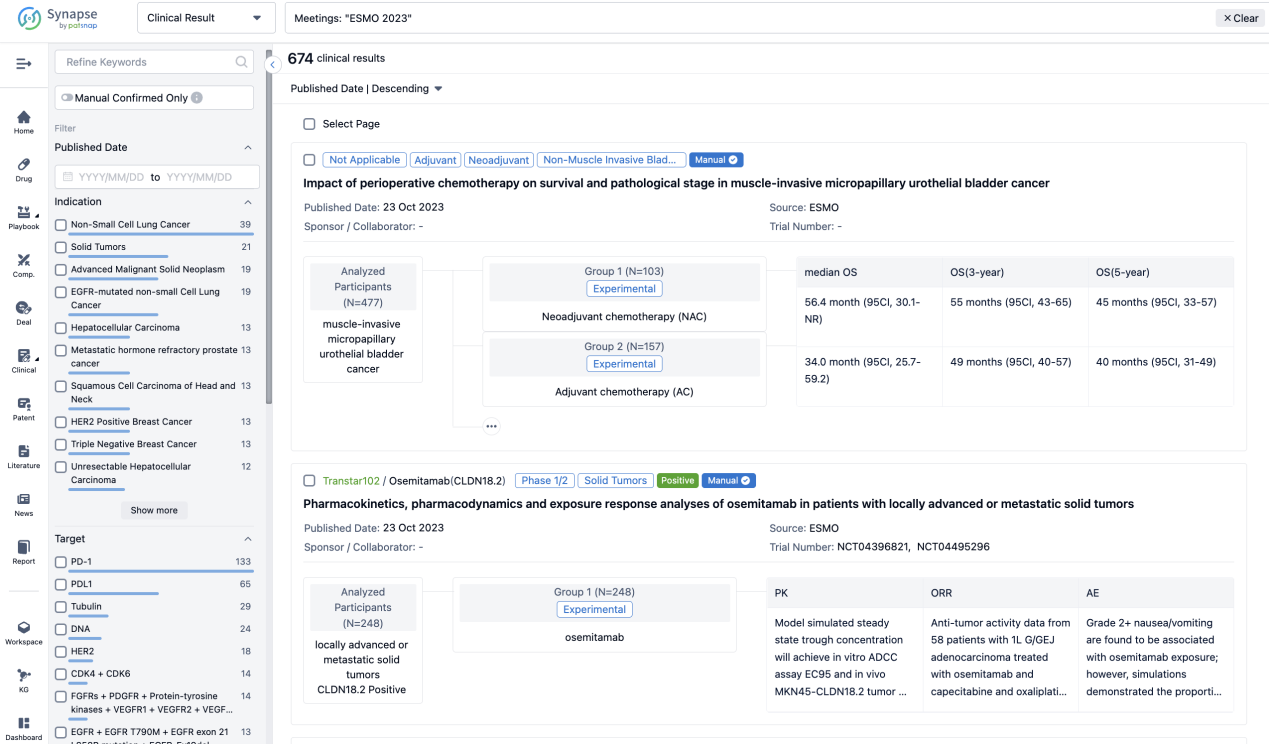
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

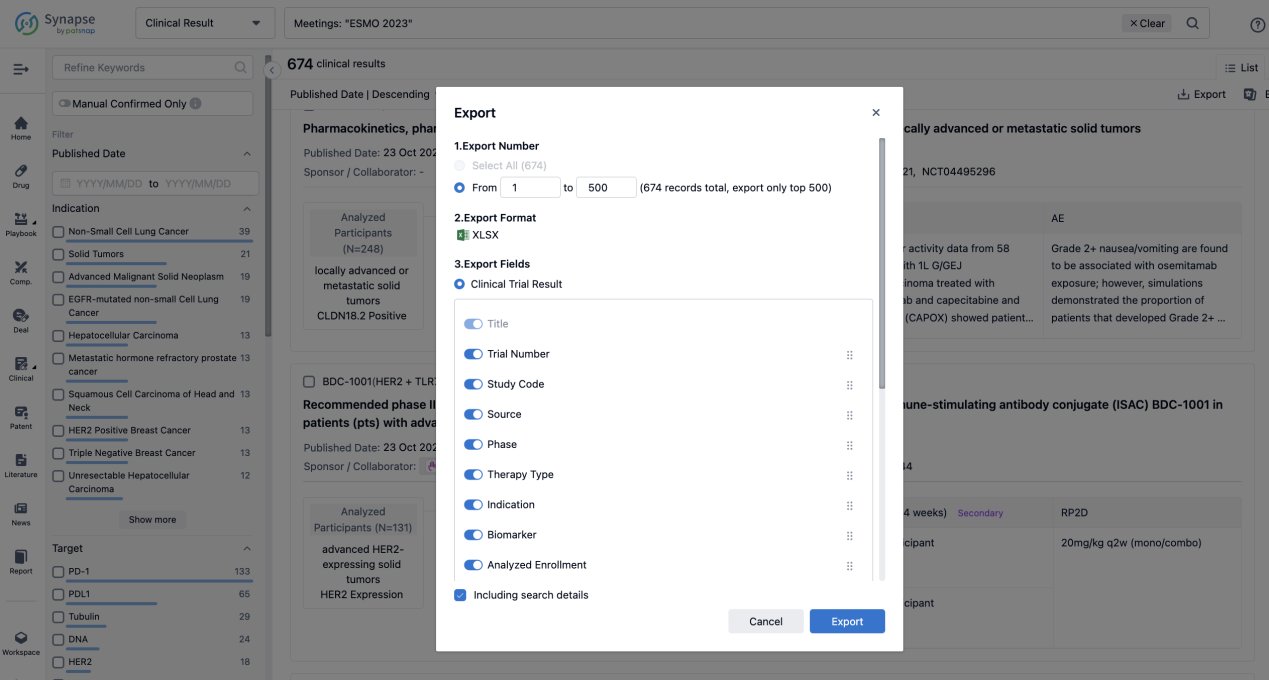
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!