An In-depth Analysis of Ezetimibe/Simvastatin's R&D Progress and Mechanism of Action on Drug Target
Ezetimibe/Simvastatin's R&D Progress
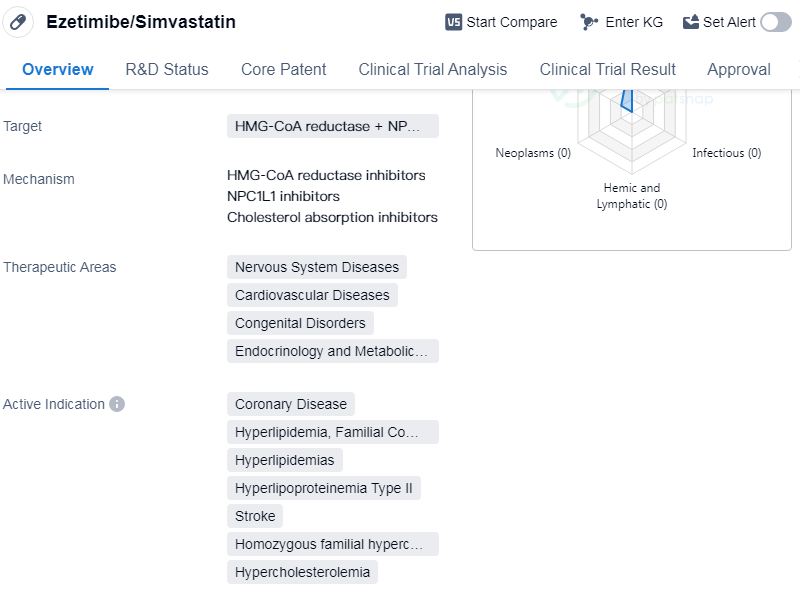
Ezetimibe/Simvastatin is a small molecule drug that targets two enzymes, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and Niemann-Pick C1-Like 1 (NPC1L1). It is used in the treatment of various therapeutic areas including nervous system diseases, cardiovascular diseases, congenital disorders, endocrinology, and metabolic diseases. The drug is indicated for the treatment of coronary disease, hyperlipidemia (familial combined), hyperlipidemia, hyperlipoproteinemia type II, stroke, homozygous familial hypercholesterolemia, and hypercholesterolemia.
The drug was developed by Merck & Co., Inc., a renowned pharmaceutical company. It has received approvals in multiple countries, with its first approval granted in Mexico in March 2004. The highest phase of development for Ezetimibe/Simvastatin is approved, indicating that it has successfully completed clinical trials and demonstrated its safety and efficacy.
Ezetimibe/Simvastatin is primarily used for the management of lipid disorders and related conditions. It works by inhibiting the production of cholesterol in the liver and reducing the absorption of cholesterol from the intestine. By targeting both HMG-CoA reductase and NPC1L1, the drug offers a dual mechanism of action to effectively lower cholesterol levels in the body.
The therapeutic areas in which Ezetimibe/Simvastatin is indicated highlight its potential in treating a wide range of diseases. Cardiovascular diseases, including coronary disease and stroke, are major causes of morbidity and mortality worldwide. Hyperlipidemia and hyperlipoproteinemia type II are conditions characterized by high levels of lipids in the blood, which can lead to the development of atherosclerosis and cardiovascular complications. Homozygous familial hypercholesterolemia is a rare genetic disorder that causes extremely high cholesterol levels and increases the risk of cardiovascular events.
The approval of Ezetimibe/Simvastatin in multiple countries, including China, indicates its global recognition and acceptance as an effective therapeutic option. Its small molecule nature allows for easy formulation and administration, making it a convenient choice for patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ezetimibe/Simvastatin: HMG-CoA Reductase Inhibitors, NPC1L1 Inhibitors, and Cholesterol Absorption Inhibitors
HMG-CoA reductase inhibitors, NPC1L1 inhibitors, and cholesterol absorption inhibitors are all types of drugs used in the treatment of high cholesterol levels.
HMG-CoA reductase inhibitors, also known as statins, are a class of drugs that work by inhibiting an enzyme called HMG-CoA reductase. This enzyme plays a crucial role in the production of cholesterol in the liver. By blocking this enzyme, statins reduce the amount of cholesterol synthesized in the body, leading to a decrease in overall cholesterol levels.
NPC1L1 inhibitors, on the other hand, target a protein called NPC1L1 that is responsible for the absorption of cholesterol from the intestine. By inhibiting NPC1L1, these drugs reduce the uptake of cholesterol from dietary sources, thereby lowering cholesterol levels in the bloodstream.
Cholesterol absorption inhibitors are a broader category of drugs that includes NPC1L1 inhibitors. These drugs work by interfering with the absorption of cholesterol in the intestine, preventing its entry into the bloodstream. By reducing the amount of cholesterol absorbed from the diet, these inhibitors help to lower overall cholesterol levels.
Overall, these drugs are used to manage high cholesterol levels, which is a risk factor for cardiovascular diseases such as heart attacks and strokes. By lowering cholesterol levels, they help to reduce the buildup of plaque in the arteries and improve heart health. However, it's important to note that these drugs are typically prescribed as part of a comprehensive treatment plan that includes lifestyle modifications such as a healthy diet and regular exercise.
Drug Target R&D Trends for Ezetimibe/Simvastatin
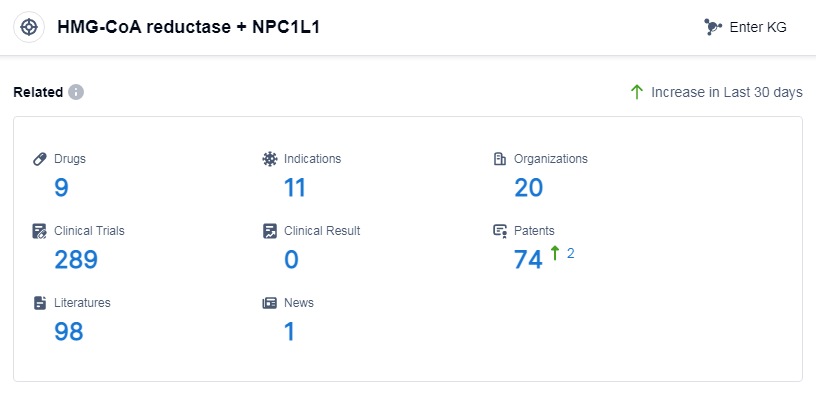
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 9 HMG-CoA reductase and NPC1L1 drugs worldwide, from 20 organizations, covering 11 indications, and conducting 289 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of the target HMG-CoA reductase and NPC1L1 shows that Merck & Co., Inc. and Organon & Co. are the leading companies with the highest stage of development. The indications for drugs under this target cover a wide range of conditions related to cholesterol and lipid metabolism. Small molecule drugs are the most rapidly progressing drug type. The United States, South Korea, Australia, and Japan are the countries/locations with the highest phase of development, with China also showing progress. Overall, the target HMG-CoA reductase and NPC1L1 have a diverse range of companies, indications, drug types, and global development, indicating a competitive landscape and potential for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Ezetimibe/Simvastatin is a small molecule drug developed by Merck & Co., Inc. It targets HMG-CoA reductase and NPC1L1 and is indicated for the treatment of various diseases related to lipid disorders. With its multiple approvals and a high phase of development, Ezetimibe/Simvastatin has demonstrated its efficacy and safety in managing cardiovascular and metabolic diseases.