An In-depth Analysis of Propantheline Bromide's R&D Progress and Mechanism of Action on Drug Target
Propantheline Bromide's R&D Progress
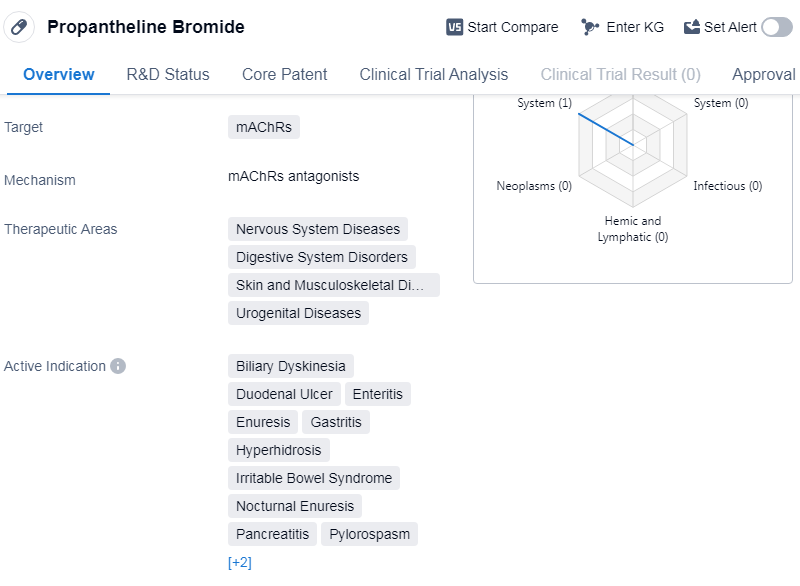
Propantheline Bromide is a small molecule drug that targets muscarinic acetylcholine receptors (mAChRs). It is used in the treatment of various nervous system diseases, digestive system disorders, skin and musculoskeletal diseases, and urogenital diseases. The drug has been approved for multiple indications and has a long history of use.
In terms of therapeutic areas, Propantheline Bromide is indicated for the treatment of biliary dyskinesia, duodenal ulcer, enteritis, enuresis, gastritis, etc.
Propantheline Bromide was first approved in the United States in April 1953, making it one of the earliest drugs in the market. The drug is a product of G.D. Searle Co., an originator organization that played a significant role in its development and commercialization.
The highest phase of development for Propantheline Bromide is approved, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization. This suggests that the drug has been thoroughly evaluated for safety and efficacy and has demonstrated positive results in treating the targeted conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Propantheline Bromide: mAChRs antagonists
mAChRs antagonists refers to muscarinic acetylcholine receptor antagonists. These are a class of drugs that block the action of acetylcholine at muscarinic receptors in the body. Muscarinic receptors are a type of cholinergic receptor found in various tissues, including the central nervous system, smooth muscles, and glands.
From a biomedical perspective, mAChRs antagonists are commonly used in the treatment of various medical conditions. For example, they are used to manage symptoms of overactive bladder by relaxing the smooth muscles of the bladder and reducing urinary urgency. They can also be used to treat certain gastrointestinal disorders by reducing excessive motility and secretion.
Additionally, mAChRs antagonists are sometimes used in anesthesia to prevent or reverse the effects of excessive acetylcholine release, which can occur during surgery or certain medical procedures. By blocking the muscarinic receptors, these drugs help maintain a balanced autonomic nervous system response.
It's important to note that mAChRs antagonists can have side effects, such as dry mouth, blurred vision, constipation, and urinary retention, due to their broader effects on muscarinic receptors throughout the body. Therefore, their use should be carefully monitored and prescribed by healthcare professionals.
Drug Target R&D Trends for Propantheline Bromide
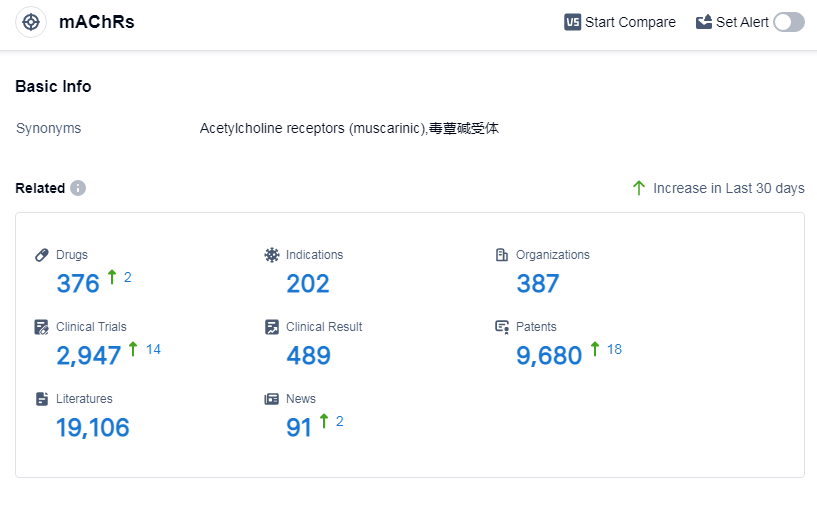
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 376 mAChRs drugs worldwide, from 387 organizations, covering 202 indications, and conducting 2947 clinical trials.
The analysis of target mAChRs reveals a competitive landscape with multiple companies actively involved in drug development. Pfizer Inc. is leading in terms of the highest number of drugs in various development phases. Indications such as pulmonary disease, chronic obstructive, urinary bladder, overactive, spasm, and asthma have seen significant drug approvals. Small molecule drugs dominate the drug types, indicating their effectiveness in targeting mAChRs. China, Japan, the United States, and the European Union are the key countries/locations driving drug development in this area. Overall, the target mAChRs present a promising area for pharmaceutical companies, with opportunities for further research and development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Propantheline Bromide is a small molecule drug that targets mAChRs and is used in the treatment of various nervous system diseases, digestive system disorders, skin and musculoskeletal diseases, and urogenital diseases. It has been approved for multiple indications and has a long history of use since its first approval in the United States in 1953. Developed by G.D. Searle Co., the drug has reached the highest phase of development and has also received approval in China.