An In-depth Analysis of Ramelteon's R&D Progress
Ramelteon's R&D Progress
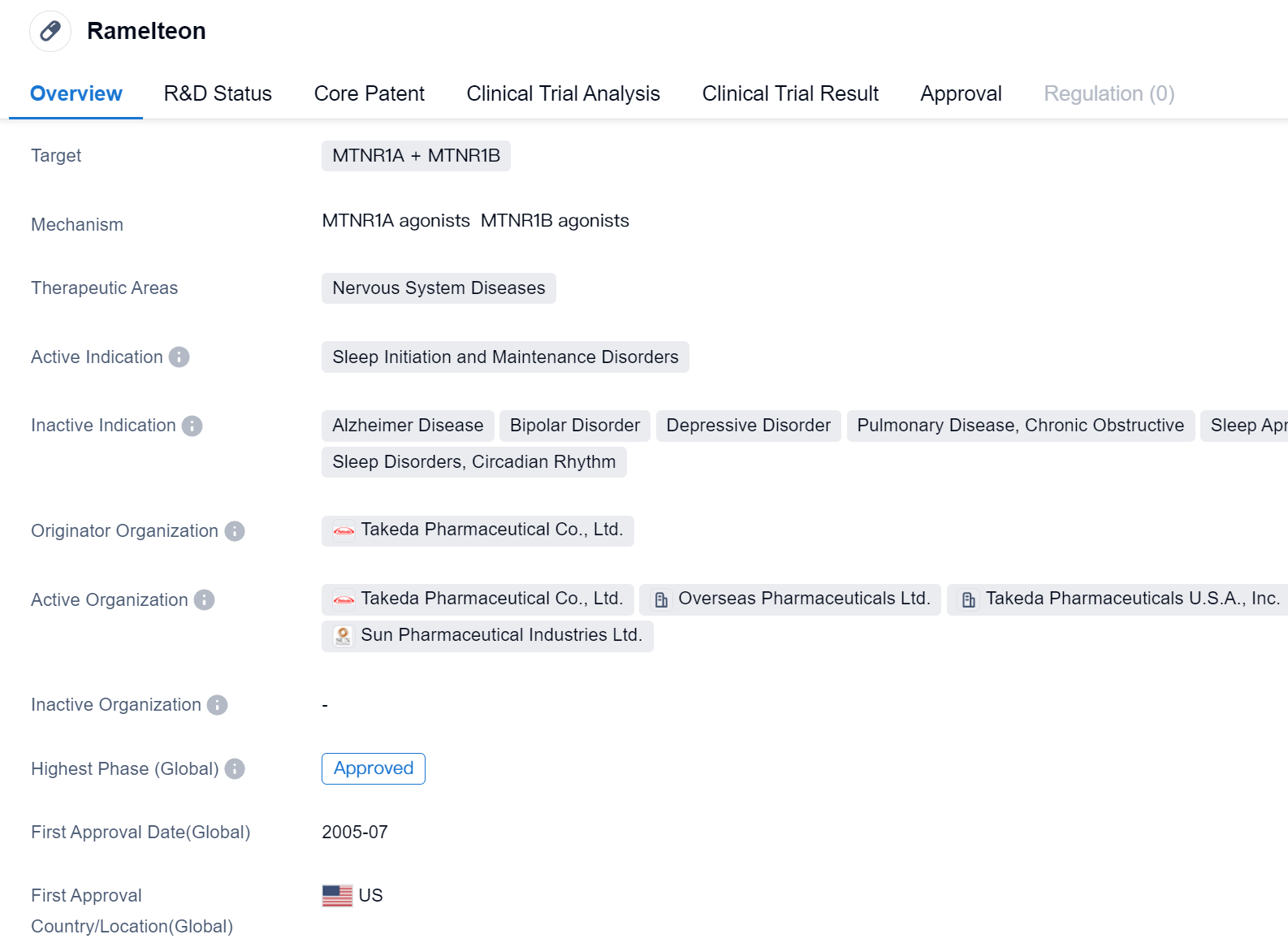
Ramelteon is a small molecule drug that targets the MTNR1A and MTNR1B receptors in the field of biomedicine. It is primarily used for the treatment of sleep initiation and maintenance disorders, which fall under the therapeutic area of nervous system diseases. The drug was developed by Takeda Pharmaceutical Co., Ltd., a renowned pharmaceutical organization.
Ramelteon has undergone extensive clinical trials and has achieved the highest phase of approval globally. It was first approved in the United States in July 2005, making it available for patients suffering from sleep disorders in that country.
As a small molecule drug, Ramelteon works by targeting the MTNR1A and MTNR1B receptors. These receptors are involved in regulating the sleep-wake cycle and are found in the brain. By targeting these receptors, Ramelteon helps to initiate and maintain sleep, providing relief to individuals struggling with sleep disorders.
The approval of Ramelteon in the United States signifies its safety and efficacy in treating sleep initiation and maintenance disorders. It has undergone rigorous testing and evaluation to ensure its effectiveness and minimize potential side effects. The drug's approval in the United States also indicates that it has met the necessary regulatory requirements set by the relevant authorities.
While Ramelteon has achieved approval in the United States, its status in China is still in the investigational phase. This suggests that further studies and evaluations are required before it can be approved for use in the Chinese market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ramelteon: MTNR1A agonists and MTNR1B agonists
MTNR1A agonists and MTNR1B agonists are compounds or substances that bind to and activate the MTNR1A and MTNR1B receptors, respectively.
From a biomedical perspective, MTNR1A and MTNR1B are subtypes of melatonin receptors. Melatonin is a hormone produced by the pineal gland in the brain that regulates the sleep-wake cycle. The MTNR1A and MTNR1B receptors are G-protein coupled receptors that are primarily found in the brain, specifically in areas involved in circadian rhythm regulation.
MTNR1A agonists are substances that specifically bind to and activate the MTNR1A receptor. By activating this receptor, these agonists mimic the effects of melatonin and can help regulate the sleep-wake cycle. They can be used in the treatment of sleep disorders, such as insomnia.
Similarly, MTNR1B agonists are substances that specifically bind to and activate the MTNR1B receptor. These agonists may have different effects compared to MTNR1A agonists, as the MTNR1B receptor is involved in various physiological processes, including glucose homeostasis and insulin release. Therefore, MTNR1B agonists may have potential applications in the treatment of metabolic disorders, such as type 2 diabetes.
In summary, MTNR1A agonists and MTNR1B agonists are compounds that selectively activate the MTNR1A and MTNR1B receptors, respectively. They can have therapeutic potential in regulating sleep-wake cycles and treating sleep disorders or metabolic disorders, depending on the specific receptor they target.
Drug Target R&D Trends for Ramelteon
MTNR1A and MTNR1B are two important receptors found in the human body that are involved in regulating the sleep-wake cycle and circadian rhythm. MTNR1A, also known as melatonin receptor 1A, is primarily expressed in the brain and plays a crucial role in mediating the effects of melatonin, a hormone that regulates sleep. MTNR1B, or melatonin receptor 1B, is found in various tissues including the brain, pancreas, and liver, and is involved in regulating glucose metabolism and insulin secretion. Both receptors are potential targets for pharmaceutical interventions aimed at treating sleep disorders, circadian rhythm disturbances, and metabolic disorders such as diabetes.
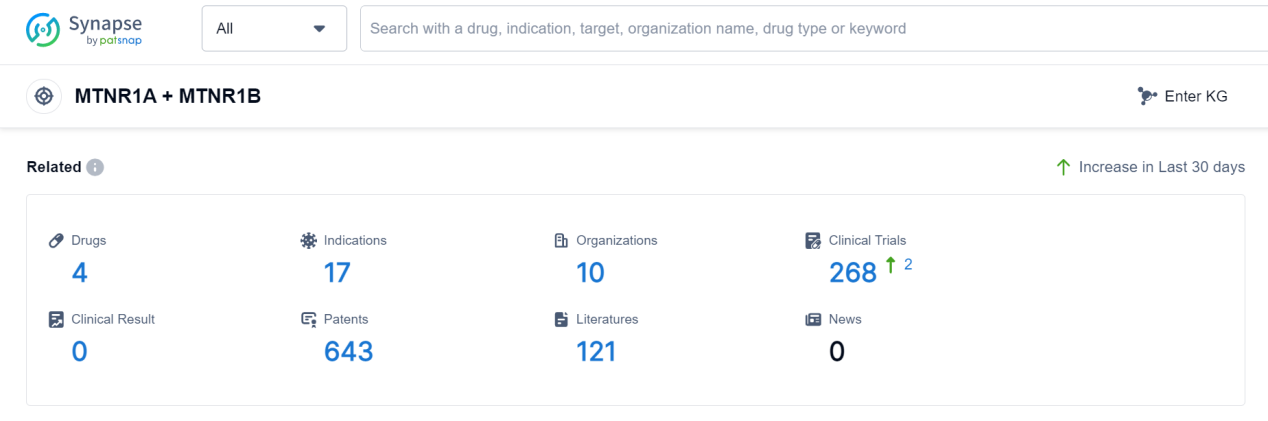
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 4 MTNR1A + MTNR1B drugs worldwide, from 10 organizations, covering 17 indications, and conducting 268 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Ramelteon is a small molecule drug developed by Takeda Pharmaceutical Co., Ltd. It targets the MTNR1A and MTNR1B receptors and is primarily used for the treatment of sleep initiation and maintenance disorders. The drug has achieved approval in the United States, indicating its safety and efficacy, while in China, it has received IND approval, signifying its ongoing evaluation and development in that market.