An In-depth Analysis of Rivastigmine Tartrate's R&D Progress and Mechanism of Action on Drug Target
Rivastigmine Tartrate's R&D Progress
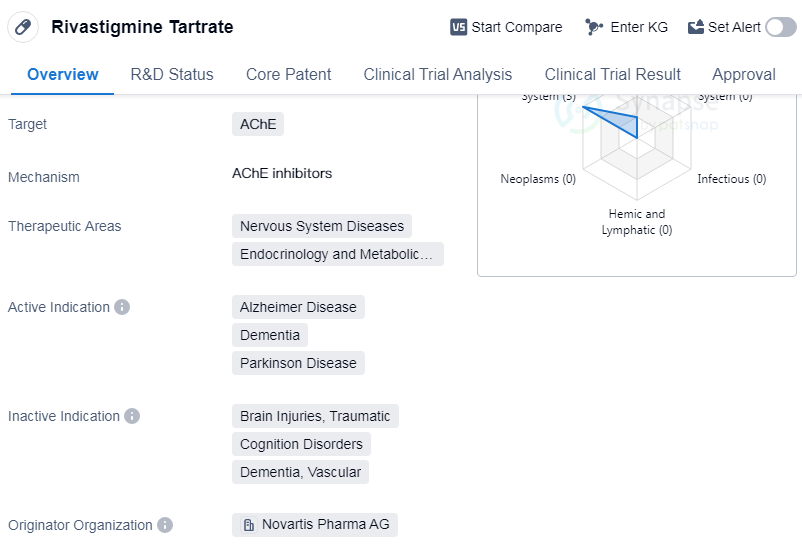
Rivastigmine Tartrate is a small molecule drug that targets the enzyme acetylcholinesterase (AChE). It is primarily used in the treatment of nervous system diseases and endocrinology and metabolic diseases. The drug has been approved for the treatment of Alzheimer's disease, dementia, and Parkinson's disease.
The originator organization of Rivastigmine Tartrate is Novartis Pharma AG, a renowned pharmaceutical company. The drug has reached the highest phase of development and has been approved globally as well as in China.
The first approval of Rivastigmine Tartrate took place in May 1998 in the European Union. This indicates that the drug has been in use for over two decades and has gained recognition for its efficacy in treating the mentioned indications.
Rivastigmine Tartrate works by inhibiting the activity of acetylcholinesterase, an enzyme responsible for breaking down acetylcholine in the brain. By inhibiting this enzyme, the drug helps to increase the levels of acetylcholine, a neurotransmitter involved in memory and cognitive function. This mechanism of action makes Rivastigmine Tartrate particularly effective in managing the symptoms of Alzheimer's disease and dementia.
The approval of Rivastigmine Tartrate in the global market highlights its significance and potential in addressing the medical needs of patients suffering from Alzheimer's disease, dementia, and Parkinson's disease. The drug's approval in China is particularly noteworthy, as it indicates its acceptance and relevance in a country with a large population and a growing healthcare market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Rivastigmine Tartrate: AChE inhibitors
AChE inhibitors refer to a class of drugs that inhibit the activity of the enzyme acetylcholinesterase (AChE). Acetylcholinesterase is responsible for breaking down the neurotransmitter acetylcholine in the synaptic cleft, which is crucial for the normal functioning of the nervous system.
In a biomedical perspective, AChE inhibitors are commonly used in the treatment of various conditions, particularly in the management of Alzheimer's disease. In Alzheimer's disease, there is a deficiency of acetylcholine, leading to cognitive decline. By inhibiting AChE, these drugs increase the levels of acetylcholine in the brain, improving cognitive function and temporarily alleviating symptoms.
AChE inhibitors can also be used in the treatment of other conditions such as myasthenia gravis, a neuromuscular disorder characterized by muscle weakness. In this case, AChE inhibitors help to enhance muscle strength by increasing the availability of acetylcholine at the neuromuscular junction.
It is important to note that AChE inhibitors can have side effects, including gastrointestinal disturbances, nausea, vomiting, and in some cases, muscle weakness. Additionally, these drugs may interact with other medications, so it is crucial to consult a healthcare professional before starting or changing any treatment involving AChE inhibitors.
Drug Target R&D Trends for Rivastigmine Tartrate
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 128 AChE drugs worldwide, from 166 organizations, covering 82 indications, and conducting 1117 clinical trials.
The analysis of the target AChE reveals that AbbVie, Inc., Shanghai Pharmaceuticals Holding Co., Ltd., Pfizer Inc., Zeria Pharmaceutical Co., Ltd., and Eisai Co., Ltd. are the companies growing fastest under this target. These companies have made significant progress in R&D, with AbbVie, Inc. having the highest number of approved drugs. The drugs targeting AChE have been approved for various indications, including neurological and gastrointestinal disorders. Small molecule drugs are the primary focus of research and development for this target, indicating their rapid progression. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target AChE, with China leading in terms of approved drugs. Overall, the competitive landscape for the target AChE is dynamic, with potential for further development and advancements in the future.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Rivastigmine Tartrate is a small molecule drug that targets AChE and is approved for the treatment of nervous system diseases and endocrinology and metabolic diseases. Its approval in multiple indications and regions, along with its long history of use, demonstrates its effectiveness and potential in the field of biomedicine.