An In-depth Analysis of Tenecteplase's R&D Progress and Mechanism of Action on Drug Target
Tenecteplase's R&D Progress
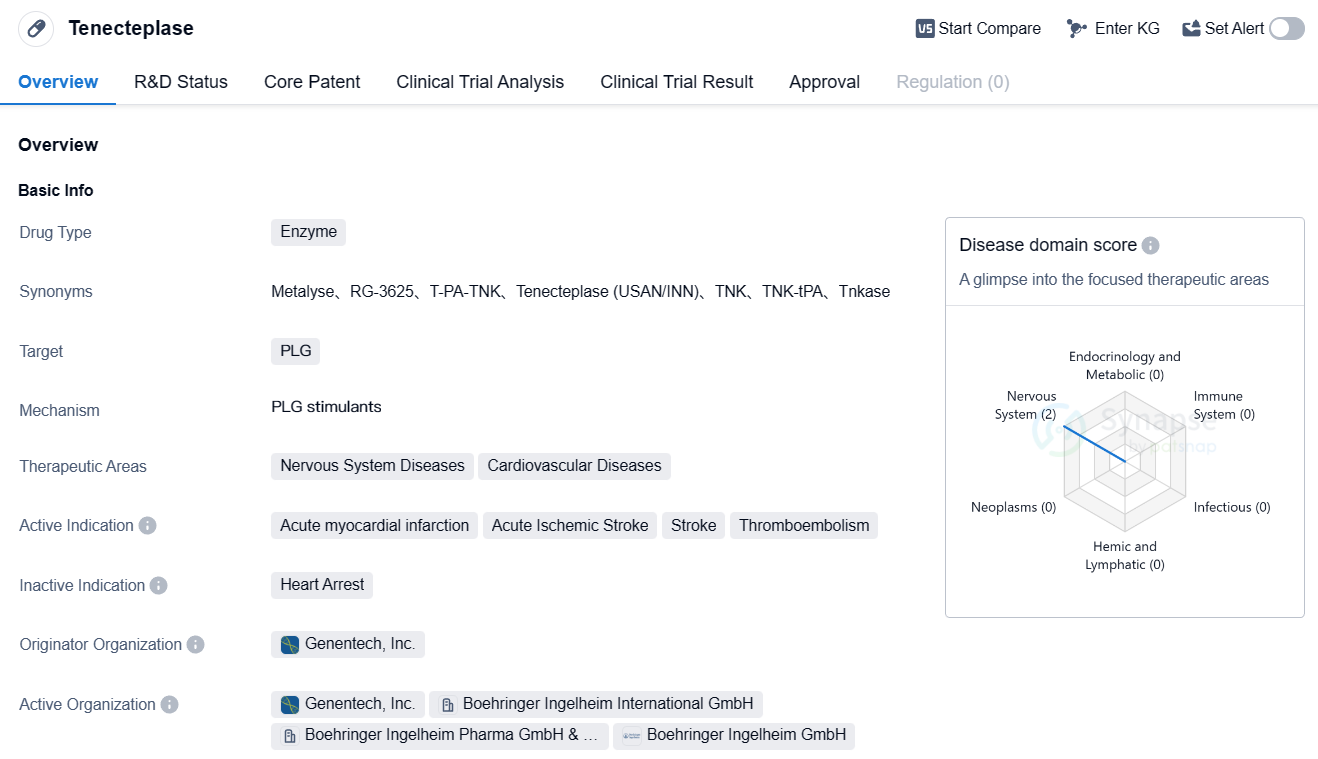
Tenecteplase is an enzyme-based drug that targets plasminogen (PLG) and is used in the treatment of various nervous system and cardiovascular diseases. It was developed by Genentech, Inc., a renowned pharmaceutical company. The drug has been approved for use in the treatment of acute myocardial infarction, acute ischemic stroke, stroke, and thromboembolism.
Tenecteplase is primarily used in the management of acute myocardial infarction, commonly known as a heart attack. It helps dissolve blood clots that are responsible for blocking the blood flow to the heart, thereby restoring normal blood circulation and preventing further damage to the heart muscle. The drug has also shown efficacy in the treatment of acute ischemic stroke, a condition caused by a blockage in the blood vessels supplying the brain. By dissolving the clot, Tenecteplase can potentially minimize the extent of brain damage and improve patient outcomes.
In addition to its applications in cardiovascular diseases, Tenecteplase has also been investigated for its potential in treating other conditions related to the nervous system. These may include neurodegenerative disorders, such as Alzheimer's disease or Parkinson's disease, although further research is required to establish its effectiveness in these areas.
Tenecteplase has undergone rigorous clinical trials, and its highest phase of development has been approved globally. It received its first approval in the United States in June 2000, making it available for patients in need of immediate treatment for acute myocardial infarction and other related conditions. The drug has also progressed to phase 3 of development in China, indicating its potential for approval and availability in the Chinese market in the near future.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tenecteplase: PLG stimulants
PLG stimulants refer to stimulant drugs that contain or utilize poly(lactic-co-glycolic acid) (PLG) as a component or delivery system. PLG is a biodegradable and biocompatible polymer commonly used in the field of biomedicine for drug delivery purposes. It is derived from lactic acid and glycolic acid, which are both naturally occurring compounds.
In the context of stimulant drugs, PLG can be used as a carrier or encapsulation material to deliver the active ingredients to the target site in a controlled and sustained manner. The PLG matrix can be designed to release the stimulant drug over a specific period, allowing for prolonged therapeutic effects.
Stimulant drugs, such as those used to treat attention deficit hyperactivity disorder (ADHD), work by increasing certain chemicals in the brain that help improve focus, attention, and impulse control. The use of PLG as a delivery system for these drugs can enhance their efficacy and provide a more controlled release, reducing the frequency of dosing and potential side effects.
Overall, PLG stimulants combine the benefits of stimulant drugs with the advantages of PLG as a drug delivery system, offering improved therapeutic outcomes and patient convenience.
Drug Target R&D Trends for Tenecteplase
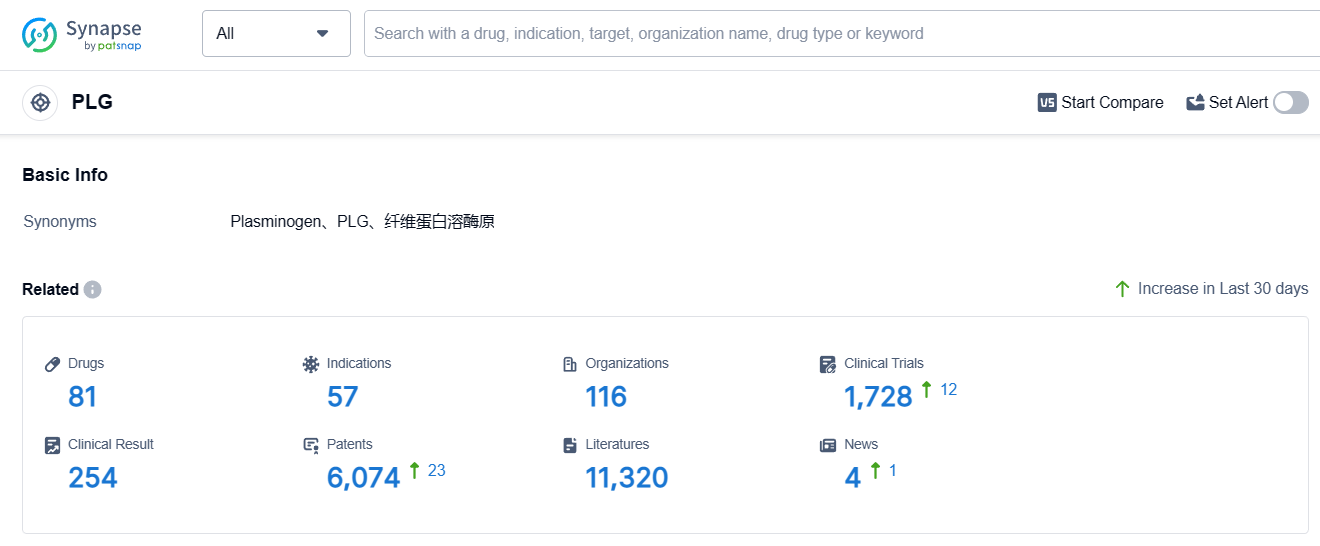
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 81 PLG drugs worldwide, from 116 organizations, covering 57 indications, and conducting 1728 clinical trials.
The current competitive landscape of the target PLG indicates that companies like Roche Holding AG, bioMérieux SA, Chandra Bhagat Pharma Ltd., Shanghai Fosun High Technology (Group) Co., Ltd., and Bharat Serums & Vaccines Ltd. are growing rapidly. The highest stage of development is the "Approved" phase, indicating successful drug development. R&D progress is evident in companies like Roche Holding AG, C.H. Boehringer Sohn AG & Co. KG, and Pfizer Inc. The approved drugs under the target PLG cover a wide range of indications, with a focus on cardiovascular diseases, thrombosis, and stroke. The drug types progressing rapidly include enzymes, biosimilars, and small molecule drugs. China, India, United States, Japan, and European Union are the countries/locations with the fastest development under the target PLG. China, in particular, has shown significant progress in terms of approved drugs and R&D activities. Overall, the target PLG presents a competitive landscape with promising future development opportunities.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tenecteplase is an enzyme-based drug developed by Genentech, Inc. It targets plasminogen and is primarily used in the treatment of acute myocardial infarction and acute ischemic stroke. It has also shown promise in the management of other nervous system diseases and cardiovascular conditions. With its global approval and ongoing development in China, Tenecteplase holds significant potential in improving patient outcomes and addressing the unmet medical needs in these therapeutic areas.