An In-depth Analysis of Tioconazole's R&D Progress and Mechanism of Action on Drug Target
Tioconazole's R&D Progress
Tioconazole is a small molecule drug that falls under the therapeutic areas of Infectious Diseases, Skin and Musculoskeletal Diseases, and Urogenital Diseases. It primarily targets fungal CYP51A1, making it effective against various fungal infections. The drug is specifically indicated for the treatment of Candidiasis, Vulvovaginal, and Nail Diseases.
Tioconazole has a long history in the pharmaceutical industry, with its first approval in the United States in February 1983. It is worth noting that the drug has reached the highest phase of approval globally. However, in China, it is currently in the NDA/BLA phase, suggesting that it is still undergoing regulatory review for approval in the country.
Candidiasis, a common fungal infection caused by Candida species, affects various parts of the body, including the mouth, throat, and genital area. Vulvovaginal candidiasis specifically refers to the infection of the vagina and vulva in women. Tioconazole's approval for these indications highlights its effectiveness in treating these conditions.
Additionally, Tioconazole is indicated for the treatment of nail diseases. Fungal nail infections, also known as onychomycosis, can cause discoloration, thickening, and brittleness of the nails. Tioconazole's approval for this indication suggests its efficacy in combating fungal nail infections.
As a small molecule drug, Tioconazole likely acts by inhibiting the fungal CYP51A1 enzyme. This enzyme is involved in the synthesis of ergosterol, an essential component of fungal cell membranes. By targeting this enzyme, Tioconazole disrupts the integrity of the fungal cell membrane, leading to the death of the fungal cells.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tioconazole: fungal CYP51A1 inhibitors
From a biomedical perspective, fungal CYP51A1 inhibitors are a type of drugs that specifically target and inhibit the enzyme known as CYP51A1 in fungi. CYP51A1 is an essential enzyme involved in the biosynthesis of ergosterol, a vital component of the fungal cell membrane. By inhibiting CYP51A1, these inhibitors disrupt the production of ergosterol, leading to impaired fungal cell membrane integrity and function.
Fungal CYP51A1 inhibitors are commonly used in the treatment of fungal infections, such as those caused by Candida species or Aspergillus species. By selectively targeting the fungal enzyme, these inhibitors have minimal impact on human CYP51A1, reducing the risk of adverse effects in patients.
It is important to note that the specific mechanisms of action, efficacy, and potential side effects can vary among different fungal CYP51A1 inhibitors. Therefore, it is crucial to consult a healthcare professional or refer to specific drug information for detailed explanations and guidance regarding their use.
Drug Target R&D Trends for Tioconazole
Fungal CYP51A1, also known as lanosterol 14α-demethylase, plays a crucial role in the synthesis of ergosterol, a vital component of fungal cell membranes. This enzyme is responsible for the demethylation of lanosterol, a precursor molecule in the ergosterol biosynthesis pathway. By inhibiting fungal CYP51A1, antifungal drugs can effectively disrupt ergosterol production, leading to membrane dysfunction and ultimately fungal cell death. Due to the absence of CYP51A1 in humans, these drugs selectively target fungal infections while minimizing adverse effects on human cells. Understanding the role of fungal CYP51A1 is essential for the development of effective antifungal therapies.
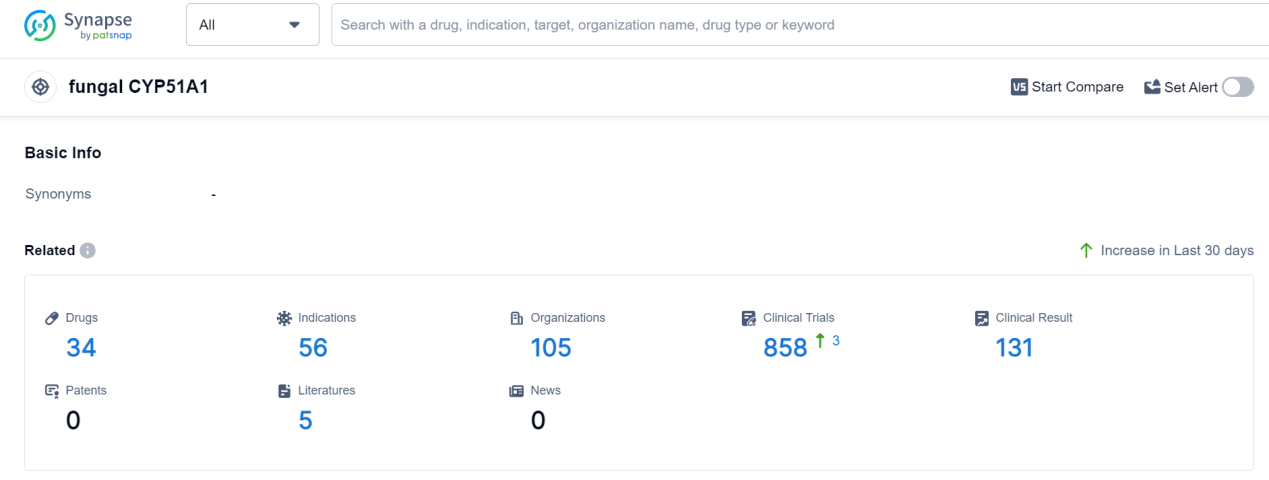
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 34 fungal CYP51A1 drugs worldwide, from 105 organizations, covering 56 indications, and conducting 858 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Tioconazole is an approved drug with a broad therapeutic scope in the field of biomedicine. Its efficacy against fungal infections, particularly Candidiasis, Vulvovaginal, and Nail Diseases, makes it a valuable treatment option. However, further regulatory processes are required for its approval in China, indicating potential expansion into new markets.