An Overview of Eisai’s Drug Pipeline | Drug Targets
Eisai, Inc. is a multinational company headquartered in Tokyo and founded in 1941 with overseas branches, subsidiaries and factories throughout Europe, America and Asia. Eisai is centered at the Tsukuba Research Institute and is engaged in research in the specialized fields of neurology, gastroenterology, and oncology. The company was first listed on the Tokyo and Osaka stock exchanges in 1961.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Eisai.
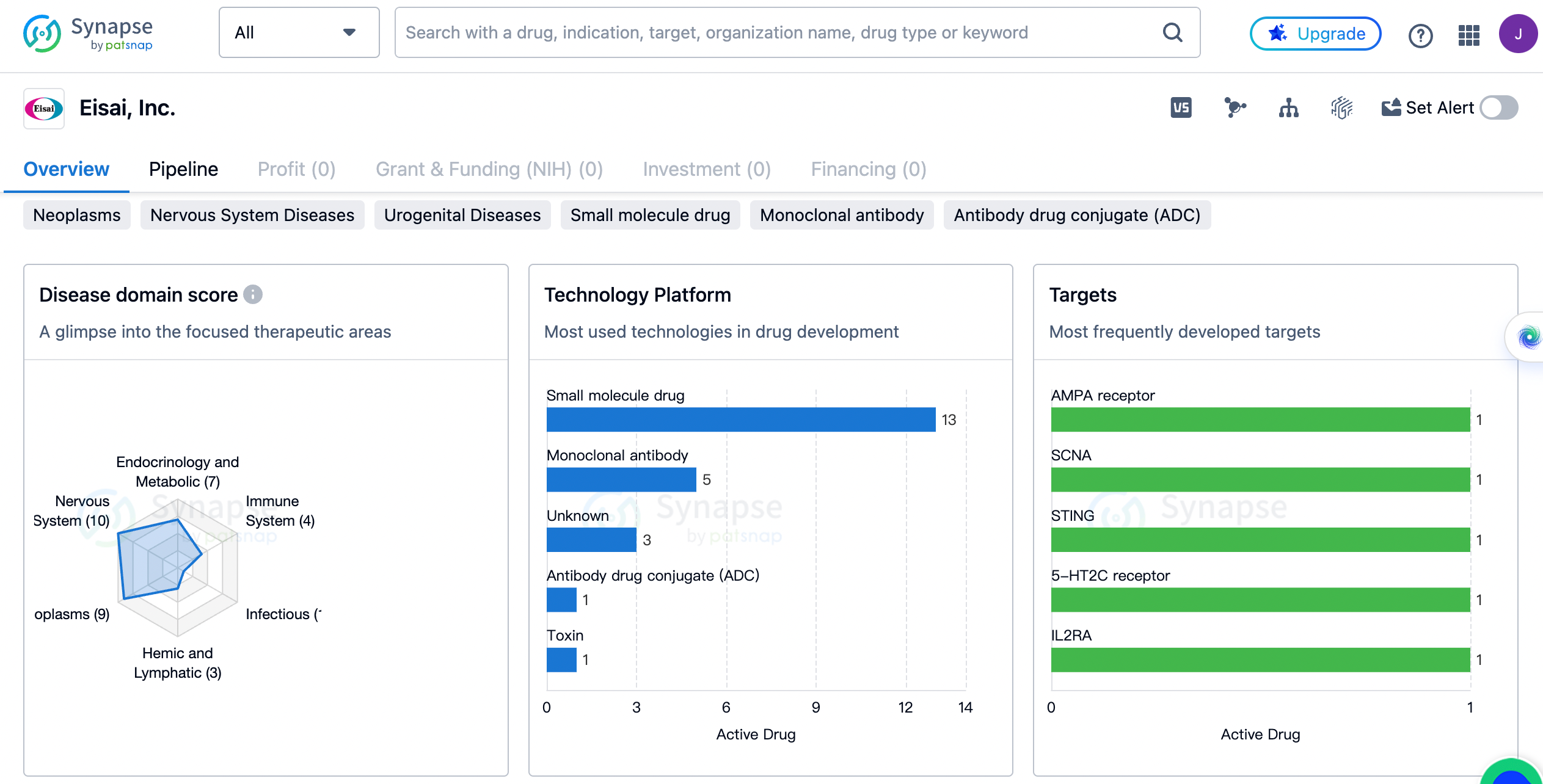
Eisai, Inc. has developed drugs for a wide range of therapeutic areas. The organization has the highest drug count in the field of Nervous System Diseases, with a total of 10 drugs. This indicates that Eisai, Inc. has a strong focus on developing treatments for neurological conditions. The second highest drug count is in the field of Neoplasms, with 9 drugs. This suggests that the organization is also actively involved in the development of cancer treatments.
In addition to Nervous System Diseases and Neoplasms, Eisai, Inc. has developed drugs for other therapeutic areas as well. These include Endocrinology and Metabolic Disease (7 drugs), Urogenital Diseases (6 drugs), Skin and Musculoskeletal Diseases (4 drugs), Immune System Diseases (4 drugs), Respiratory Diseases (4 drugs), Digestive System Disorders (3 drugs), Hemic and Lymphatic Diseases (3 drugs), Congenital Disorders (2 drugs), Cardiovascular Diseases (1 drug), Other Diseases (1 drug), and Infectious Diseases (1 drug).
The most frequently developed targets by Eisai
The targets include AMPA receptor, SCNA, STING, 5-HT2C receptor, IL2RA, CREBBP + CTNNB1, AChE, TLR7 + TLR8, Tubulin, Orexin receptor, FGFR1 + FGFR2 + FGFR3 + FGFR4 + PDGFRα + RET + VEGFR1 + VEGFR2 + VEGFR3 + c-Kit, PDE9A, APP, cGMP-PDE, FOLR1, MSLN + Tubulin, TAU, and FOLR1 + Tubulin. Each target is associated with the development of one drug, indicating that Eisai, Inc. has a diverse range of drug targets.
An overview of Eisai's pipeline
The pipeline is categorized into different phases of drug development. Eisai, Inc. has 3 drugs in the Preclinical phase, indicating that they are in the early stages of development. There are no drugs in the IND (Investigational New Drug) phase or IND Approval phase.
In terms of clinical development, Eisai, Inc. has 5 drugs in Phase 1, 3 drugs in Phase 2, and 2 drugs in Phase 3. This indicates that the organization has a significant number of drugs in advanced stages of clinical trials. However, there are no drugs in the NDA/BLA (New Drug Application/Biologics License Application) phase. The pipeline also includes 9 drugs that have received approval, indicating that Eisai, Inc. has successfully brought these drugs to market. Additionally, there are 25 drugs categorized under "Other," which could include drugs in various stages of development or those that do not fit into the traditional phases of drug development.
In summary, Eisai, Inc. is a pharmaceutical organization that was founded in 1995 and is based in New Jersey, United States. The company focuses on the development and distribution of drugs for various therapeutic areas. They have a strong presence in the field of Nervous System Diseases and Neoplasms, with a significant number of drugs developed for these areas. Eisai, Inc. also targets a diverse range of drug targets. Their pipeline includes drugs in various stages of development, with a significant number of drugs in advanced clinical trials. Overall, Eisai, Inc. has a diverse portfolio of drugs and a promising pipeline for future growth in the pharmaceutical industry.