An Overview of Genmab’s Drug Pipeline | Therapeutic Areas
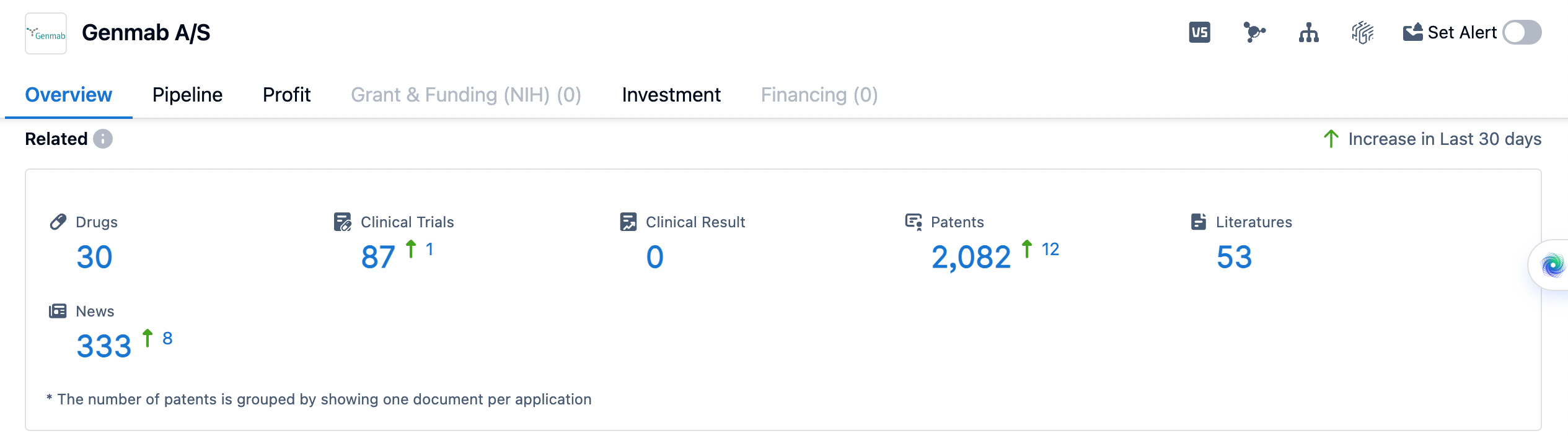
Genmab A/S is a biopharmaceutical company based in Hovedstaden, Denmark. It was founded in 1999 and has since become a prominent player in the pharmaceutical industry. The company focuses on the development of innovative antibody-based therapies for the treatment of various diseases. Genmab currently has two approved antibodies: Arzerra® (ofatumumab) for certain chronic lymphocytic leukemia indications and DARZALEX™ (daratumumab) for the treatment of severe double-refractory multiple myeloma. Genmab is also involved in multiple pre-clinical pipeline projects and has four proprietary antibody R&D platform technologies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Genmab.
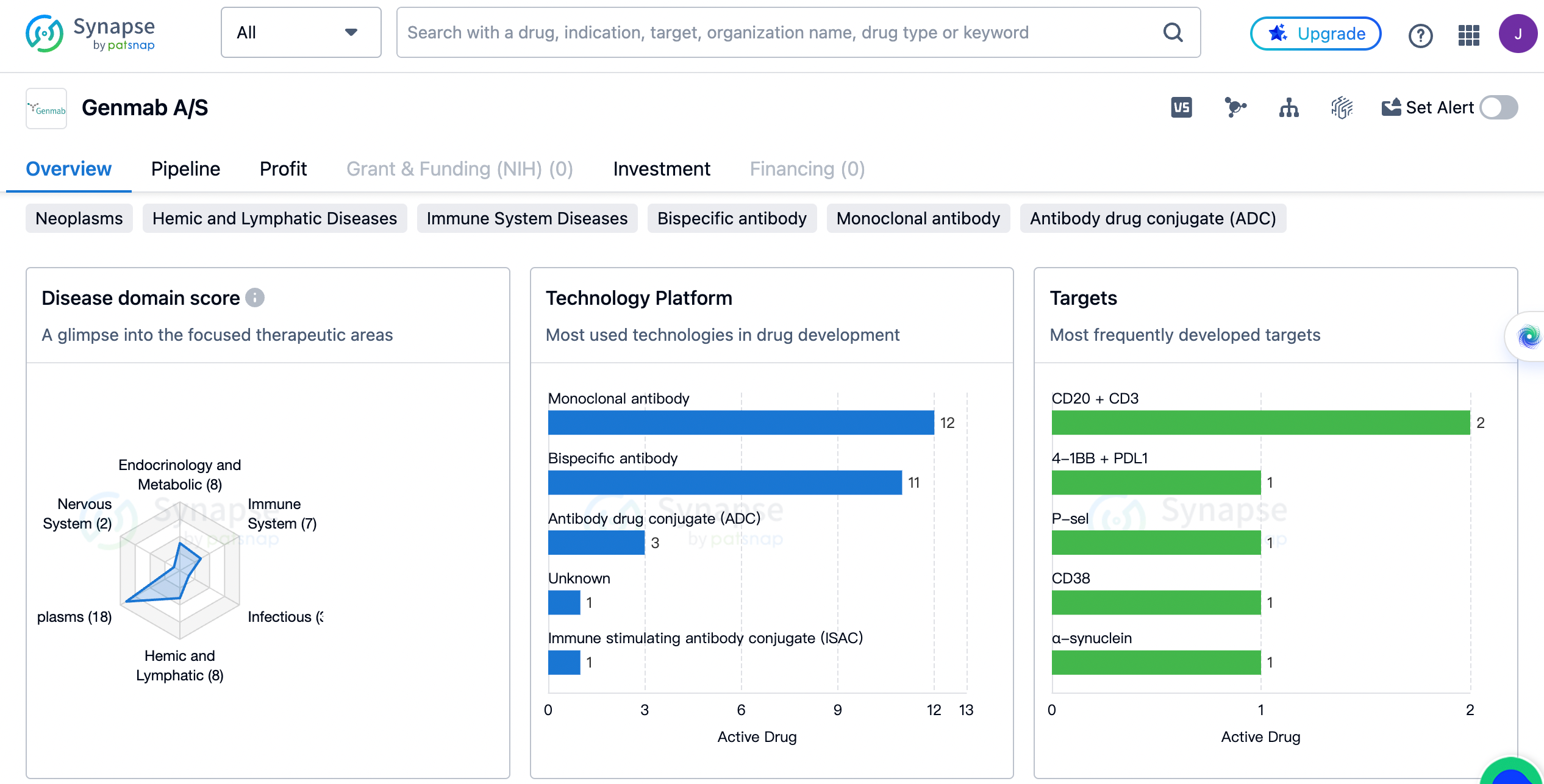
In terms of therapeutic areas, Genmab A/S has a diverse portfolio. The company has developed drugs for a wide range of diseases, including neoplasms, respiratory diseases, hemic and lymphatic diseases, endocrinology and metabolic diseases, digestive system disorders, skin and musculoskeletal diseases, immune system diseases, urogenital diseases, cardiovascular diseases, infectious diseases, congenital disorders, and nervous system diseases. Among these, neoplasms have the highest drug count with 18 drugs, followed by respiratory diseases with 9 drugs. The remaining therapeutic areas have drug counts ranging from 8 to 1.
The most frequently developed targets by genmab
When it comes to the most frequently developed targets by Genmab A/S, the company has focused on a variety of molecules and proteins. These targets include CD20 + CD3, 4-1BB + PDL1, P-sel, CD38, α-synuclein, IL2RA, DR5, IL-8, 5T4 + CD3, EGFR + c-Met, CD37, 4-1BB + CD40L, F10 + factor IXa, IL-15, AXL, CD276, tissue factor, AXL + Tubulin, B7-H4 + CD3, and Tubulin + tissue factor. Each of these targets has been associated with the development of at least one drug by Genmab A/S.
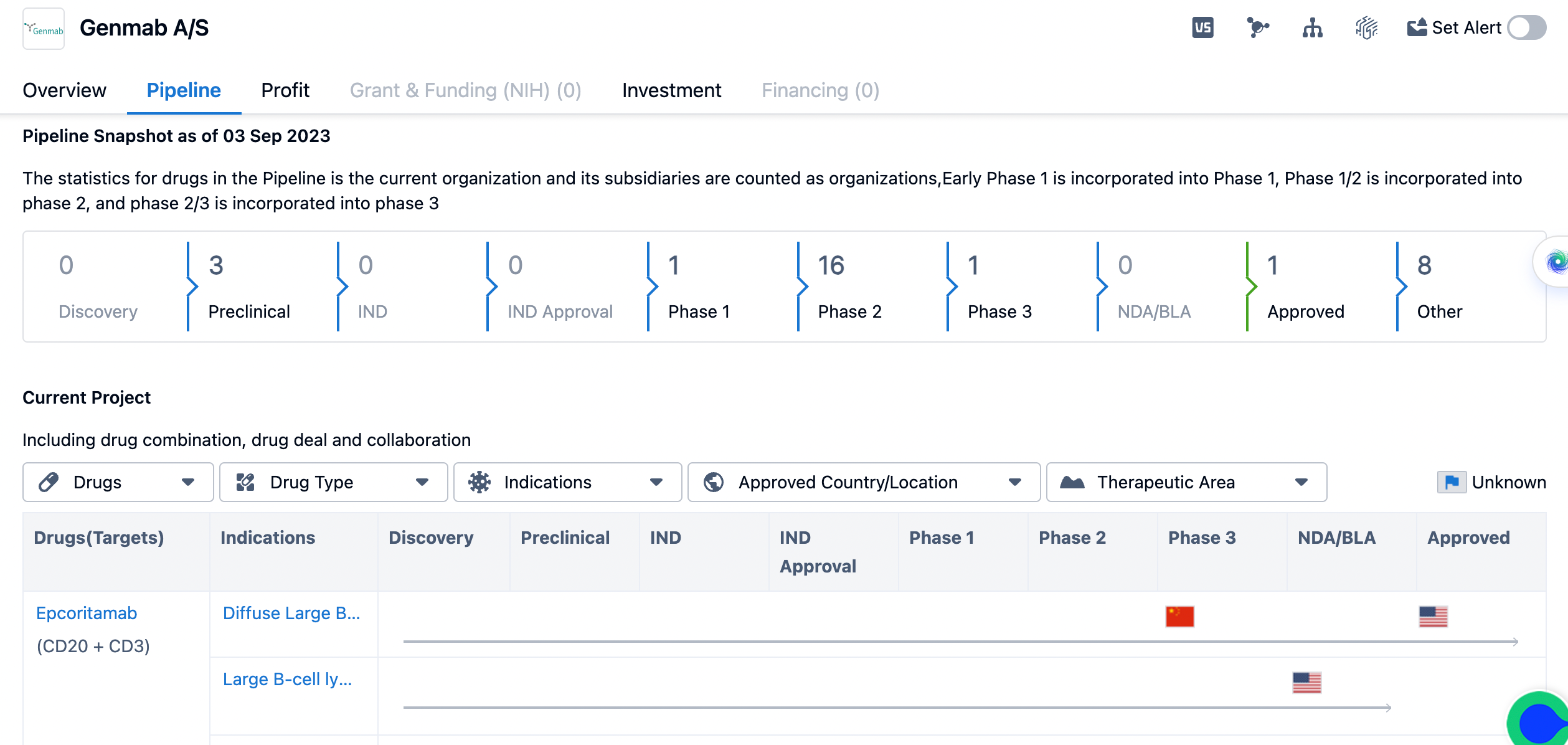
The pipeline of genmab
Looking at the pipeline of Genmab A/S, it is evident that the company has a robust development program. As of September 3, 2023, the pipeline consists of drugs in various stages of development. The majority of drugs are in Phase 2, with a count of 16. This indicates that these drugs have shown promising results in early clinical trials and are now being further evaluated for their safety and efficacy. Additionally, there is one drug in Phase 1, one drug in Phase 3, and one drug that has already been approved. The remaining drugs are in preclinical stages or categorized as "Other," which could include drugs in early discovery or post-approval studies.
Genmab A/S has demonstrated a strong commitment to research and development, as evidenced by its pipeline. The company's focus on antibody-based therapies aligns with the growing trend in the pharmaceutical industry towards targeted and personalized treatments. Antibodies have shown great potential in treating various diseases, including cancer and autoimmune disorders, due to their ability to specifically target disease-causing molecules.
The therapeutic areas targeted by Genmab A/S reflect the company's dedication to addressing unmet medical needs. Neoplasms, or tumors, have the highest drug count, indicating the company's emphasis on developing innovative cancer therapies. This aligns with the global need for effective and targeted treatments for various types of cancer. Respiratory diseases, hemic and lymphatic diseases, and endocrinology and metabolic diseases also have a significant drug count, highlighting the company's efforts to tackle these prevalent health issues.
The diverse range of targets developed by Genmab A/S demonstrates the company's commitment to exploring different mechanisms of action and therapeutic approaches. By targeting molecules and proteins involved in various disease pathways, Genmab A/S aims to develop therapies that can effectively modulate disease progression and improve patient outcomes.
In conclusion, Genmab A/S is a leading biopharmaceutical company with a strong focus on antibody-based therapies. The company's pipeline showcases its dedication to research and development, with a significant number of drugs in advanced stages of clinical development. The therapeutic areas and targets chosen by Genmab A/S reflect the company's commitment to addressing unmet medical needs and developing innovative treatments for a wide range of diseases. With its continued efforts in the pharmaceutical industry, Genmab A/S is poised to make significant contributions to the field of biomedicine.