The world's first Maternal Vaccine! Analysis of Pfizer's RSV Vaccine
On August 21, Pfizer announced: The U.S. Food and Drug Administration (FDA) approved its Respiratory Syncytial Virus (RSV) bivalent vaccine, Abrysvo, for use in pregnant women between 32-36 weeks of gestation, to prevent lower respiratory tract infections caused by RSV in infants from birth up to 6 months old.
This is the first and only RSV vaccine for pregnant women, marking an important milestone for public health.
Pfizer anticipates that the vaccine will officially launch in the third quarter of 2023.
RSV is a common infectious virus that affects the lungs and the respiratory tract. Statistics show that the global annual prevalence of RSV is approximately 3-10%, with about 102,000 children dying from RSV infection each year. Moreover, the elderly are also susceptible to RSV due to the decline in immunity and underlying diseases caused by aging, resulting in a high risk of severe RSV-related diseases.
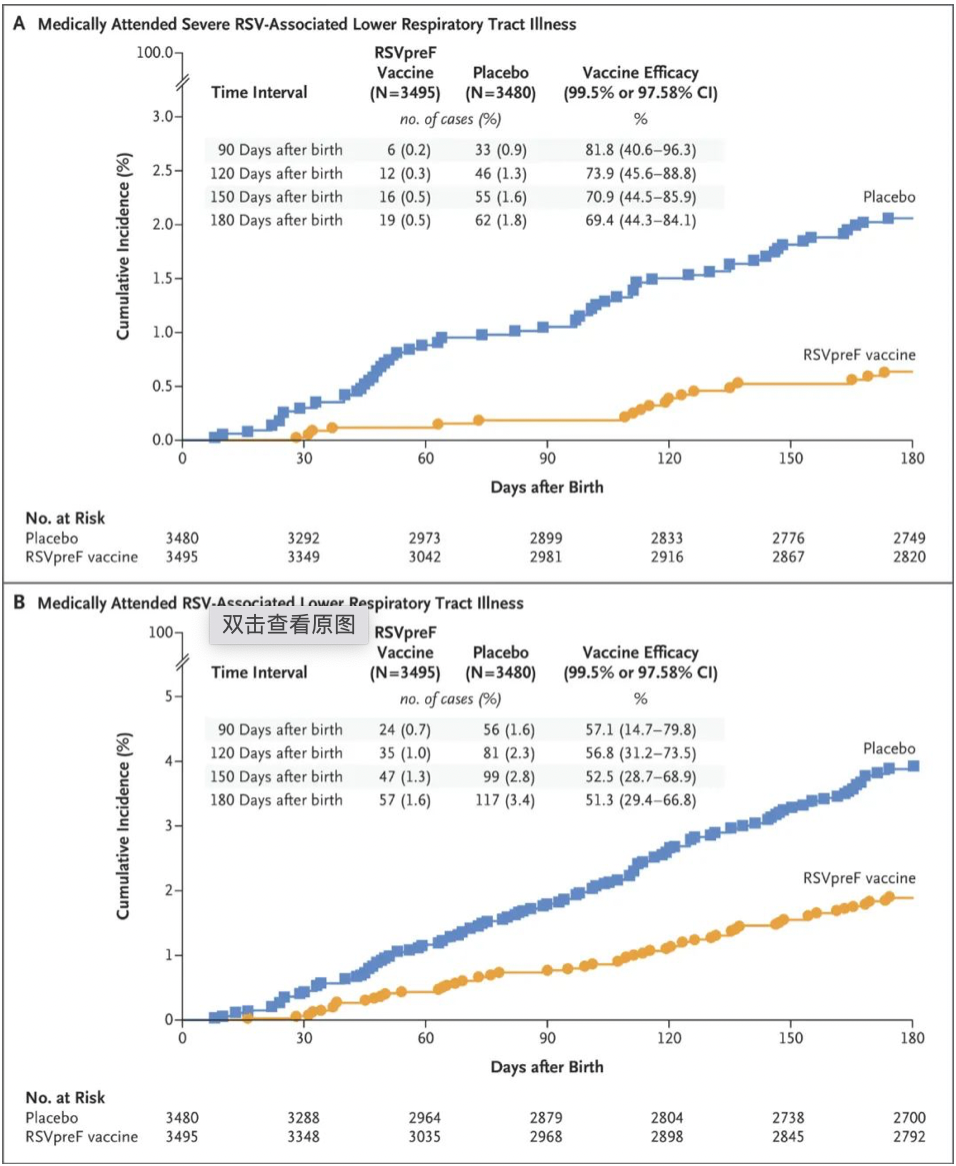
The BLA is primarily based on the positive key results of the Phase III MATISSE study. The study included 7,400 pregnant women trial subjects, with an aim to evaluate the effectiveness, safety, and immunogenicity of RSVpreF in preventing RSV-induced medically-attended lower respiratory tract infections (MA-LRTI) and severe MA-LRTI.
The interim analysis shows that in the first 90 days after the baby's birth, the protection efficacy of RSVpreF against severe MA-LRTI reached 81.8%, and it was 69.4% during the follow-up period of 6 months, significantly better than the placebo group. The protection efficacy against MA-LRTI was 57.1%, with a 6-month follow-up efficacy of 51.3%, showing no significant differential to the placebo group. The vaccine was well-tolerated, and no safety issues were observed in the vaccinated pregnant women or their newborns.
Abrysvo Approval Progress
On March 2, 2022, the U.S. Food and Drug Administration (FDA) granted Abrysvo Breakthrough Therapy Designation for use in actively immune prevention of Respiratory Syncytial Virus (RSV) related lower respiratory tract diseases in infants from birth to six months through maternal immunity.
In December 2022, the FDA awarded Abrysvo a Priority Review Designation for the prevention of lower respiratory tract diseases caused by RSV in individuals aged 60 and above.
In February 2023, the FDA once again granted Priority Review Designation to this vaccine, being able to generate active immunity through pregnant women and thus preventing infants from RSV-associated lower respiratory tract diseases from birth to six months old.
On May 31, 2023, Pfizer announced that the U.S. FDA had approved its RSV vaccine Abrysvo for marketing, to prevent acute respiratory disease and lower respiratory tract disease caused by RSV in adults aged 60 and above.
Apart from the two approved indications, Pfizer has initiated two new Phase III clinical trials for PF-06928316, aimed at evaluating the safety, tolerability, and immunogenicity of the vaccine for the prevention of RSV infection in adults at high risk for severe RSV, and in children at high risk for RSV aged between 2 and 18 years old.