Analysis on the Clinical Research Progress of SHP2 Inhibitors
From the perspective of therapeutic interventions, proteins involved in tyrosine phosphorylation in signal transduction pathways are considered potential targets in drug development, as these proteins transmit various growth factor signals during the occurrence and development of tumors. PTPN11 is the first confirmed oncogene in the tyrosine phosphatase family, playing a crucial role in signal transduction downstream of growth factor receptors. The Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP2), encoded by the PTPN11 gene, is the only confirmed oncogenic protein. Increased expression or mutations of SHP2 can lead to various solid tumors, such as leukemia, breast cancer, colon cancer, and melanoma. Since SHP2 is a regulator of proliferative signaling pathways (e.g., Ras/MAPK, PI3K/Akt), dysregulation of these pathways may lead to tumorigenesis.
Research shows that 35% of Juvenile Myelomonocytic Leukemia (JMML) carries PTPN11 mutations, mainly located in the N-SH2 and PTP domains, which may impair the self-inhibition function of SHP2, leading to persistent activation of SHP2 protein. Expression of activated mutant forms of SHP2, such as D61Y and E76K, in hematopoietic cells promotes cell cycle progression and survival of hematopoietic progenitor cells, and results in sustained activation of PI3K/Akt and Ras/MAPK. In addition to JMML, SHP2 mutants have also been found in myeloid tumors, such as Acute Myeloid Leukemia, Chronic Myelomonocytic Leukemia, Myelodysplastic Syndromes, Acute Lymphoblastic Leukemia, primarily among children, and are rarely found in adults.
The expression of SHP2 is upregulated to 70% in ductal invasive breast cancer, and the SHP2-binding protein GAB2 is also overexpressed in 10-15% of human breast cancers. Downregulation of the SHP2 gene can significantly inhibit the proliferation of breast tumor initiating cells and block the maintenance and progression of breast cancer. SHP2 levels are also often elevated in melanoma, with higher levels of expression closely related to poorer prognosis and a metastatic phenotype. Moreover, inhibiting SHP2 can suppress the growth of RTK-driven cancer cells, such as EGFR-L858R-driven lung adenocarcinoma, BRAF-mutated colon cancer, and KRAS-mutated breast cancer. In addition, in KRAS-mutated non-small cell lung cancer (NSCLC), inhibiting SHP2 can promote tumor aging, thereby triggering the immune system to eliminate cancer cells. Furthermore, analysis of tumor samples from advanced EGFR mutation-positive NSCLC patients revealed that those with higher SHP2 and ILK expression benefitted less from EGFR-TKI treatment than those with lower levels.
As a multifunctional protein, SHP2 plays a critical role in the regulation of Ras/MAPK and PI3K/Akt signaling pathways and in maintaining body homeostasis. SHP2 also has some non-signaling functions such as mitochondrial activity and epigenetic regulation. Corresponding to its multifunctionality, mutants of SHP2 can cause a range of diseases, among which the specific molecular mechanisms are still to be studied and many problems remain to be solved.
SHP2 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 25 Sep 2023, there are a total of 34 SHP2 drugs worldwide, from 54 organizations, covering 30 indications, and conducting 67 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.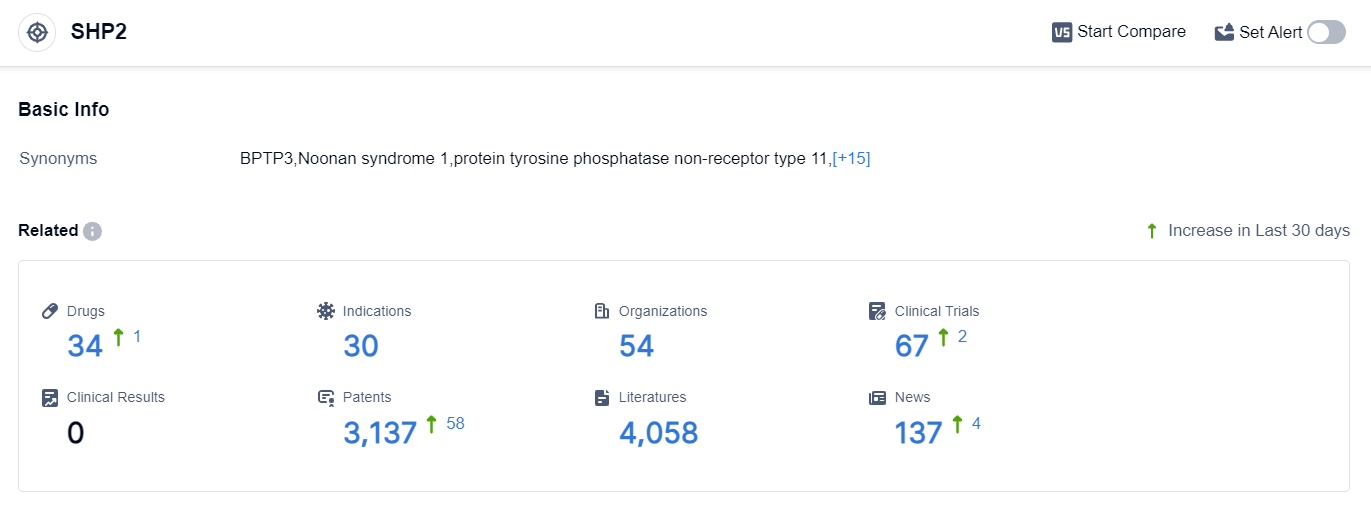
In conclusion, the target SHP2 has attracted the attention of several pharmaceutical companies, including Jacobio Pharmaceuticals Group Co., Ltd., Novartis AG, and Revolution Medicines, Inc. These companies are actively involved in the research and development of drugs targeting SHP2, with a focus on indications such as Non-Small Cell Lung Cancer, Solid Tumors, and Squamous Cell Carcinoma of Head and Neck.
The development of SHP2-targeting therapies is primarily driven by small molecule drugs and PROTACs. Small molecule drugs have shown significant progress in Phase 2, Preclinical, and Inactive stages, indicating their potential as innovative treatments. PROTACs, a novel drug class, are also gaining attention in the development of SHP2 inhibitors.
The United States, China, and Australia are leading the development of SHP2-targeting therapies, with notable progress in each country. China, in particular, has made significant strides with 2 drugs in Phase 2 and 6 drugs in Preclinical stages, showcasing its growing capabilities in the pharmaceutical industry.
Overall, the current competitive landscape of target SHP2 is characterized by active research and development efforts from various companies and countries. The future development of SHP2-targeting therapies holds promise, especially in the areas of small molecule drugs, PROTACs, and the contributions of China to the field.
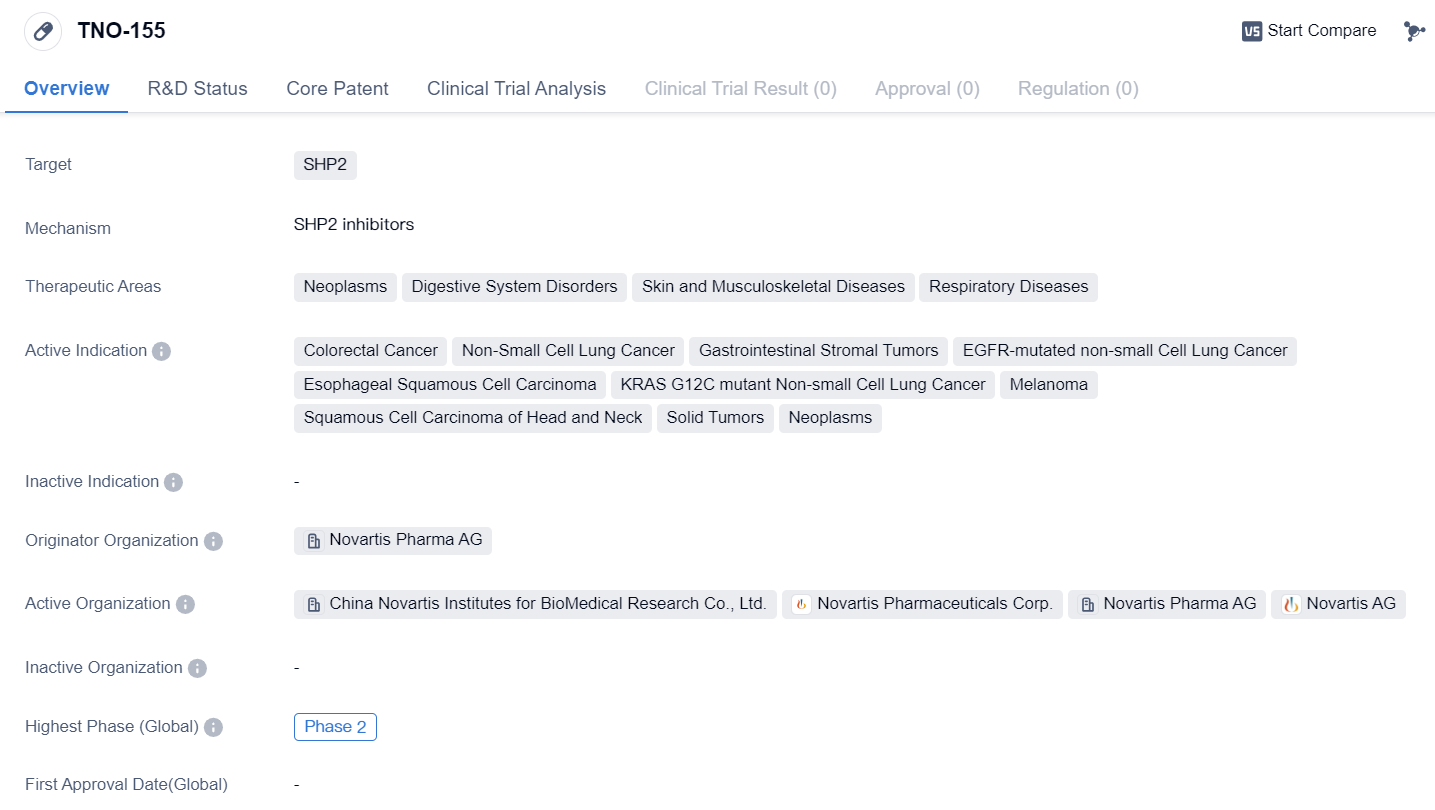
SHP2 inhibitors that have entered Phase II clinical trials: TNO-155
TNO-155 is a small molecule drug that targets SHP2, a protein involved in various cellular processes. It has shown potential therapeutic benefits in several therapeutic areas including neoplasms, digestive system disorders, skin and musculoskeletal diseases, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of specific indications, TNO-155 has demonstrated activity against colorectal cancer, non-small cell lung cancer, gastrointestinal stromal tumors, EGFR-mutated non-small cell lung cancer, esophageal squamous cell carcinoma, KRAS G12C mutant non-small cell lung cancer, melanoma, squamous cell carcinoma of the head and neck, solid tumors, and neoplasms. This wide range of indications suggests that TNO-155 may have a broad spectrum of activity against different types of cancers and other diseases.
TNO-155 is developed by Novartis Pharma AG, a renowned pharmaceutical company known for its expertise in developing innovative drugs. As of the latest available information, TNO-155 has reached Phase 2 of clinical development globally. In China, it has progressed to Phase 1, indicating that it is still in the early stages of clinical testing in this region.
In summary, TNO-155 is a small molecule drug targeting SHP2, with potential therapeutic applications in neoplasms, digestive system disorders, skin and musculoskeletal diseases, and respiratory diseases. It has shown activity against various indications, including colorectal cancer, non-small cell lung cancer, and melanoma. Developed by Novartis Pharma AG, TNO-155 has reached Phase 2 globally and Phase 1 in China, indicating ongoing clinical development and potential future advancements in its therapeutic use.
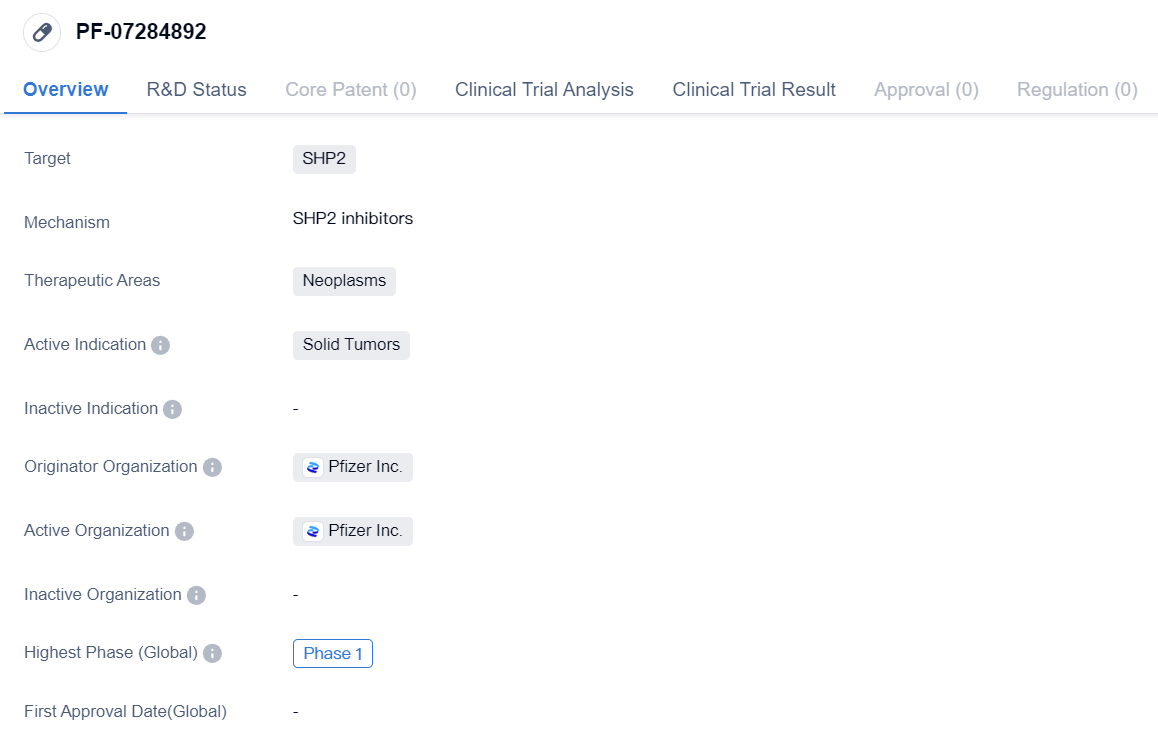
SHP2 inhibitors that have entered Phase I clinical trials: PF-07284892
The drug PF-07284892 is a small molecule drug that targets SHP2, a protein involved in cell signaling pathways. It is being developed for the treatment of neoplasms, specifically solid tumors. The drug is being developed by Pfizer Inc., a leading pharmaceutical company.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, PF-07284892 is in Phase 1 of clinical development. Phase 1 trials are the first stage of testing in humans and are primarily focused on evaluating the safety and dosage of the drug. This suggests that PF-07284892 is still in the early stages of development and has not yet been tested extensively in patients.
It is worth noting that the information provided does not specify the specific indications or types of solid tumors that PF-07284892 is being evaluated for. Therefore, it is unclear whether the drug is being developed for a broad range of solid tumors or if it is targeting a specific subtype.
In terms of regulatory progress, PF-07284892 has received IND (Investigational New Drug) approval in China. IND approval is a significant milestone in the drug development process as it allows for the initiation of clinical trials in the country. This suggests that Pfizer Inc. has obtained the necessary regulatory clearance to conduct clinical trials with PF-07284892 in China.
Overall, based on the information provided, PF-07284892 is a small molecule drug being developed by Pfizer Inc. for the treatment of solid tumors. It is currently in Phase 1 of clinical development and has received IND approval in China. However, further information is needed to fully understand the potential of PF-07284892 in treating neoplasms and its specific indications within the solid tumor category.