Antengene Declares Phase I Trial for ATG-031, an Anti-CD24 Monoclonal Antibody
Leading commercial-stage global biopharmaceutical innovator, Antengene Corporation Limited, committed to the discovery, development, and commercialization of top-of-the-range or pioneering solutions for hematology and oncology, has made public that the Institutional Review Board of The University of Texas MD Anderson Cancer Center in Houston, Texas, USA, has given the green light to a Phase I trial of its superior anti-CD24 antibody, ATG-031.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The clinical research, labeled as the PERFORM trial and under the supervision of MD Anderson, will focus on individuals with advanced solid tumors or B-NHL. ATG-031 will be utilized in this pioneering human multi-center, open-label, Phase I dose discovery study. This trial's primary intent is to assess ATG-031's safety, tolerability, and the optimal dose for future Phase II experiments. The research's secondary aim is to comprehend the substance's pharmacological behavior, gauge its immunogenicity, and preliminarily determine its efficacy.
Dr. Amily Zhang, Chief Medical Officer at Antengene, expressed enthusiasm for the strides ATG-031 is making. "Our intent is to delve deeper into the safety, tolerability, and early efficacy of ATG-031, and we will initiate patient enrollment for the research at the earliest opportunity. Preliminary study findings are slated for release in 2024," she said.
Dr. Jay Mei, the Founder, Chairman, and CEO of Antengene, also spoke about the progress made with ATG-031. "In just three years, our R&D team has successfully launched the ATG-031 program into a clinical phase, an accomplishment that brings us a great sense of pride." Based on the promising preclinical outcomes for ATG-031, he added, "we have strong confidence in the drug's continuing therapeutic potential being reflected in clinical studies.".
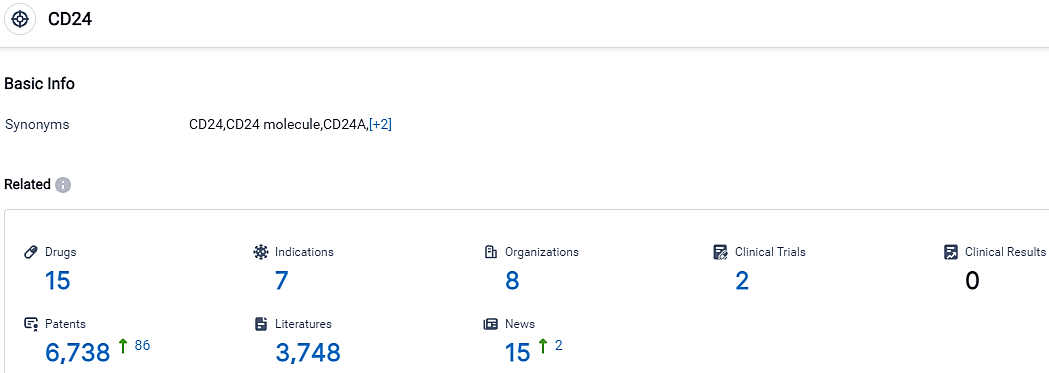
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 15 investigational drugs for the CD24 target, including 7 indications,8 R&D institutions involved, with related clinical trials reaching 2,and as many as 6738 patents.
ATG-031, a ground-breaking humanized CD24 monoclonal antibody, acts by blocking the "don't eat me" signal and increasing the ability of macrophages to engulf cancer cells. CD24 operates as a unique innate immune checkpoint and aids immune system avoidance through its engagement with the inhibitory receptor Siglec-10, found on tumor-associated macrophages. In 2023, preclinical studies showcased at the AACR revealed ATG-031's capability to attach itself specifically to CD24 with nM affinity, and interfere with CD24 and Siglec-10 interaction.