ASC Therapeutics Administers Initial Dose to Patient Using Advanced ASC618 Gene Therapy for Treating Hemophilia A
ASC Therapeutics, an independent biotech firm at the forefront of innovating in vivo gene therapy, gene modification, and allogeneic cellular treatments for blood-based and uncommon diseases, has reported the inaugural administration to a patient in their Phase I/IIa study for their prime drug, ASC618, conducted at Arkansas Children's Hospital.
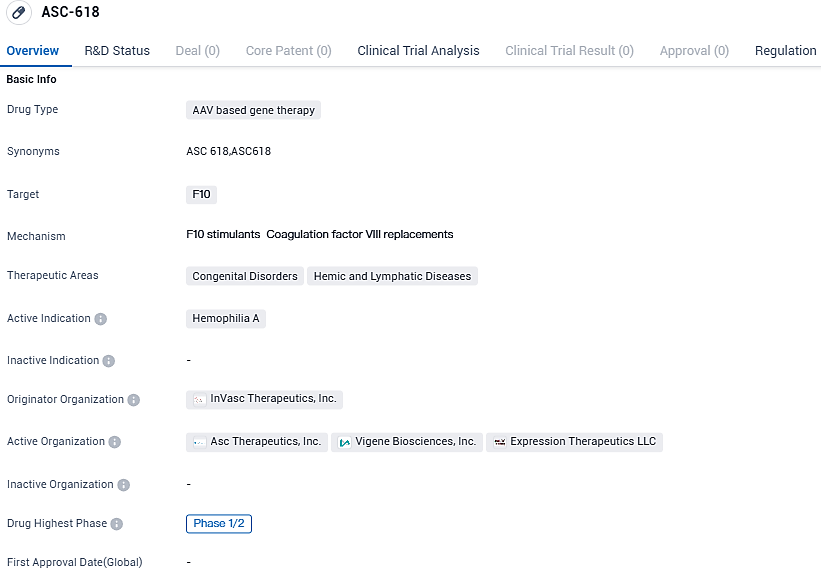
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ASC618 is a novel second-generation investigational gene therapy targeted at severe to moderately severe hemophilia A patients. The innovative Adeno-Associated Virus construct in ASC618 is composed of a uniquely designed B-domain deleted, codon-optimized, engineered chimeric Factor VIII gene, along with a concise liver-specific promoter. In preclinical trials, this construct has been able to deliver therapeutically significant quantities of FVIII protein at varying dosages.
Speaking on this occasion, Oscar Segurado, MD, PhD, and the Chief Medical Officer at ASC Therapeutics, expressed, "Today marks a remarkable milestone for ASC Therapeutics and for those living with hemophilia A who are in search of a long-lasting, cost-effective second-generation gene therapy. I am deeply appreciative of the dedication from our team and the clinical research staff at Arkansas Children’s Hospital for the successful administration to our initial patient."
In addition, Ruhong Jiang, PhD, CEO of ASC Therapeutics, mentioned, "Administering the first dose to a patient is a clear indication of our dedication to the hemophilia A community as well as our team's expertise in bringing pioneering gene-based treatments to the clinical phase."
Finally, Shelley Crary, MD, the lead researcher for the ASC618 phase I/IIa clinical study, commented, "The administration of ASC618 to our first patient reinforces our commitment to advancing state-of-the-art treatment options for individuals with hemophilia A. We are currently evaluating in a clinical context the potential of a revolutionary single-treatment gene therapy that could obviate the need for a lifetime of complex and costly therapeutic approaches to control hemophilia A."
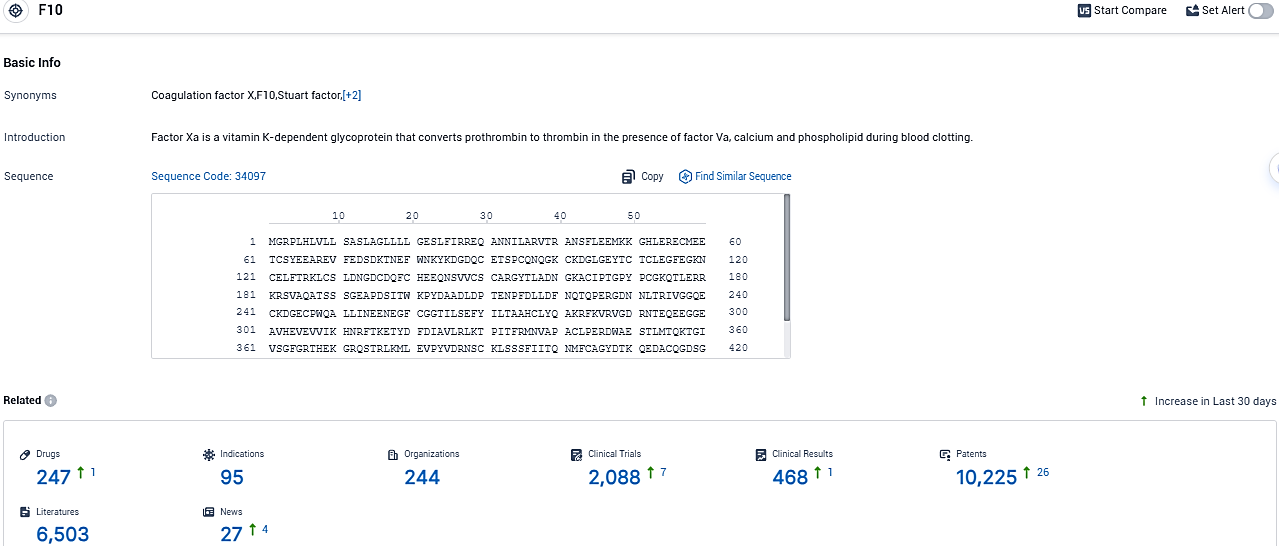
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 7, 2023, there are 247 investigational drugs for the F10 target, including 95 indications, 244 R&D institutions involved, with related clinical trials reaching 2088, and as many as 10225 patents.
ASC618 is an AAV8-based gene therapy product incorporating a novel liver-specific promoter and a bioengineered, codon-optimized B domain-deleted FVIII variant. ASC Therapeutics has obtained exclusive global rights from Expression Therapeutics to develop ASC618 and has conducted IND-enabling studies in multiple animal models that further demonstrated enhanced FVIII secretion from ASC618.