Asha Therapeutics Announces ASHA-624, a Novel Treatment Candidate for ALS
Asha Therapeutics, a biotech firm focused on innovating new therapeutic agents intended to alter the progression of neurodegenerative conditions lacking sufficient treatment options, has declared the selection of a compound in development, referred to as ASHA-624. This promising therapeutic agent, which is aimed at inhibiting the SARM1 pathway, is being advanced as a possible treatment that could adjust the course of Amyotrophic Lateral Sclerosis. Moreover, it holds potential for application in several other areas of significant clinical concern such as Chemotherapy-Induced Peripheral Neuropathy, Glaucoma, and injuries affecting the brain and spinal cord resulting from trauma.
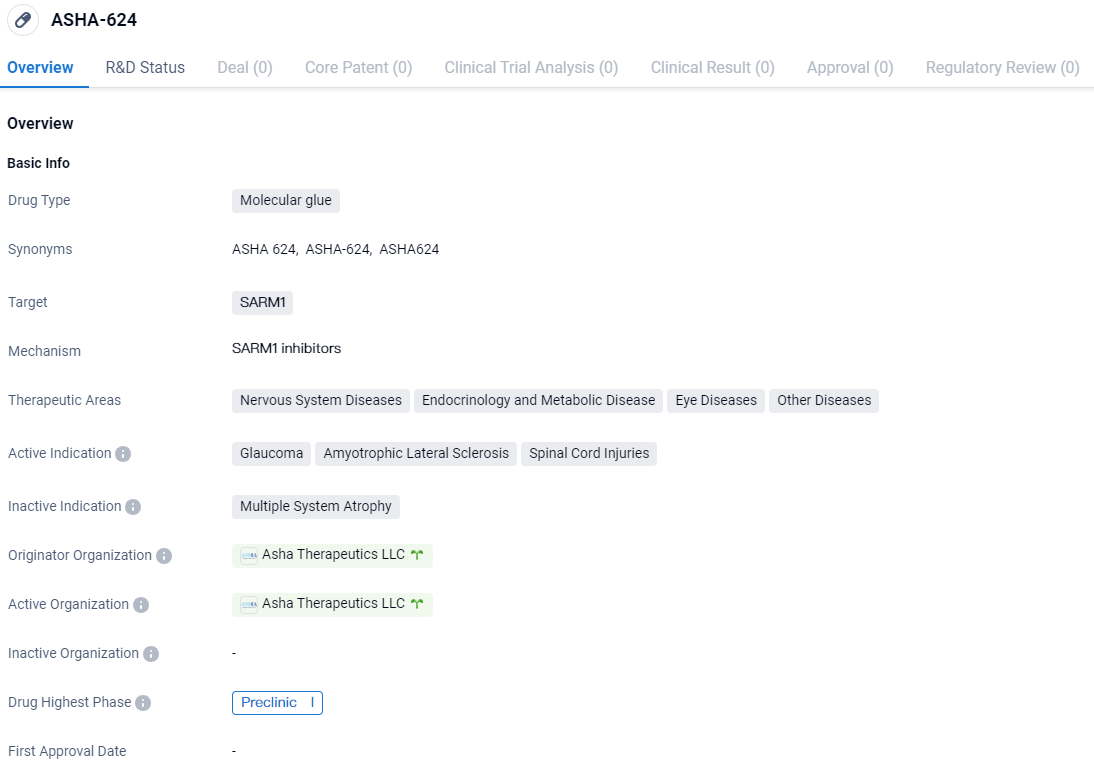
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Following a highly positive presentation by Dr. Bradlee Heckmann, Chief Scientific Officer and Scientific Co-founder, earlier in the month on the potent effectiveness and safety profile of the company's secondary neurology endeavor, ASHA-091, aimed at tackling Alzheimer’s and Parkinson’s diseases, the company made an announcement. Dr. Heckmann's discourse occurred during the prestigious AD/PD 2024 International Conference held in Lisbon, Portugal.
Dr. Michael Gold, MD, MS, an esteemed member of the Asha Scientific Advisory Board, observed, “The therapeutic target SARM-1 has a strong validation and presents an opportunity to craft new treatments for patients enduring various neurological conditions, including those of both central and peripheral origins. ASHA-624 introduces a pioneering mechanism to hinder SARM-1 activation, offering the potential for a potent clinical therapy with minimal undesirable impacts. With extensive experience in CNS drug development, I am eagerly anticipating ASHA-624's voyage into clinical study phases.”
Dr. Heckmann weighed in, “Introducing ASHA-624 as a prospective clinical development candidate for ALS, coupled with our previously selected ASHA-091, marked for Alzheimer’s, Parkinson’s diseases, and ALS itself, signifies a pivotal event for Asha. It aligns with our commitment to develop groundbreaking therapies that revamp the management of conditions which, until now, have been confined mainly to symptomatic care."
ASHA-624 stands as an innovative entity in its class that employs the natural mechanisms of SARM1, an influential protein inciting axonal damage and neuronal death. This intra-molecular glue-like compound meticulously inactivates SARM1 by binding, thus significantly preserving nervous tissue by obstructing the loss of axons and neurons.
In ALS pathology, SARM1's activation is culpable for axonal deterioration, leading to neuron death and progressive motor disability. The creation of ASHA-624 aims to precisely target and incapacitate SARM1. Preclinical experiments have underscored its considerable safety credentials, acting as a functional remedy in ALS models. Treatment with ASHA-624 in ALS animal models has restored motor capabilities nearly to the baseline observed in unaffected controls, whilst the placebo groups continued to demonstrate a steep worsening in their motor skills.
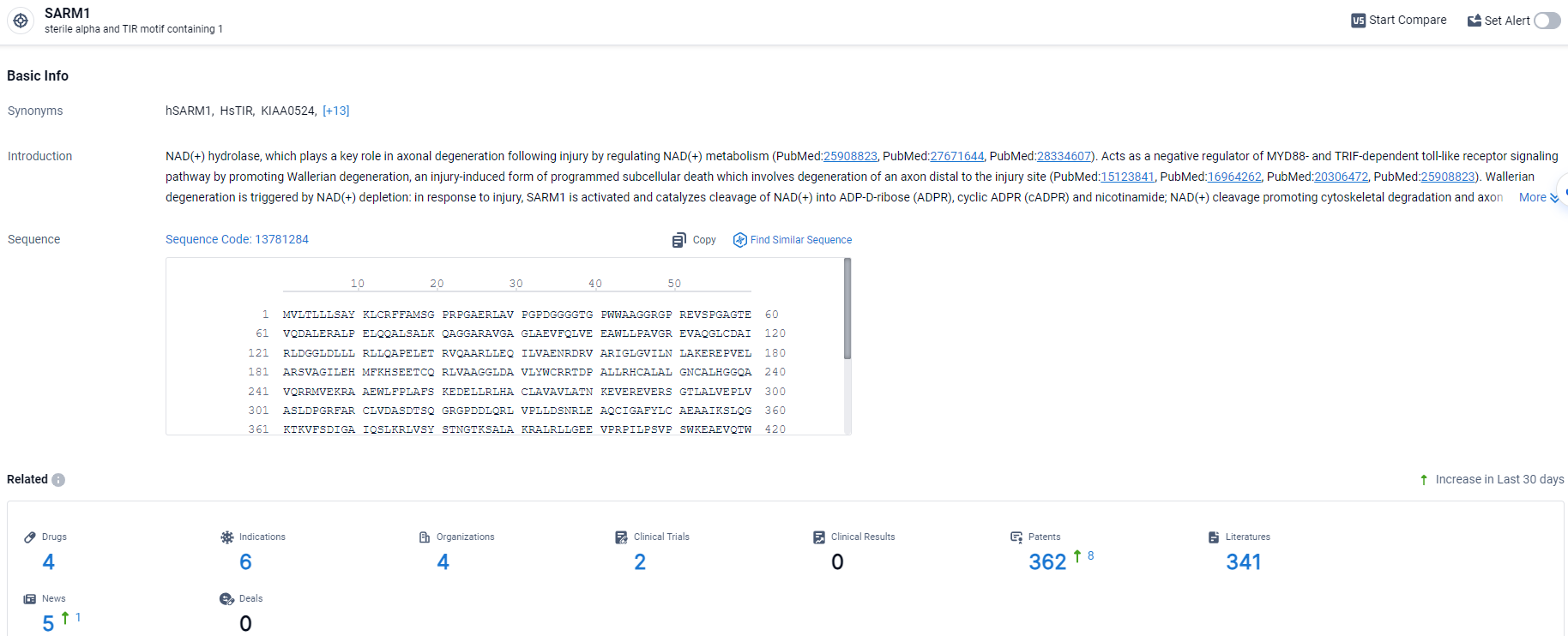
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 3, 2024, there are 4 investigational drugs for the SARM1 target, including 6 indications, 4 R&D institutions involved, with related clinical trials reaching 2, and as many as 362 patents.
ASHA-624 is a molecular glue drug that targets SARM1. It has shown potential in treating various diseases, including glaucoma, ALS, and spinal cord injuries. Further research and clinical trials will be necessary to determine its safety and efficacy in humans.