ATA3219 New Drug Application Filed by Atara for Lupus Kidney Treatment
Atara Biotherapeutics, Inc., is at the forefront of T-cell immunotherapy, utilizing its groundbreaking allogeneic Epstein-Barr virus T-cell technology to craft groundbreaking treatments for individuals suffering from oncological and autoimmune conditions. The company has recently declared that it has filed an Investigational New Drug (IND) application with the U.S. Food and Drug Administration to evaluate the efficacy of ATA3219 as a standalone treatment option targeting lupus nephritis, a manifestation of systemic lupus erythematosus impacting the kidneys.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Despite progress in treatment options, the medical needs of many lupus nephritis patients remain unaddressed, with standard treatments and currently approved medications offering limited benefits, which generally require extensive, even lifelong, immunosuppression," stated Rajani Dinavahi, Atara's Chief Medical Officer.
"We are committed to pushing the boundaries of healthcare with pioneering cellular treatments that can have a real impact. We are eager to engage with the FDA to begin this new research and work toward introducing ATA3219 in a clinical setting to offer a promising new therapeutic path for individuals battling this long-term illness," further commented Rajani Dinavahi.
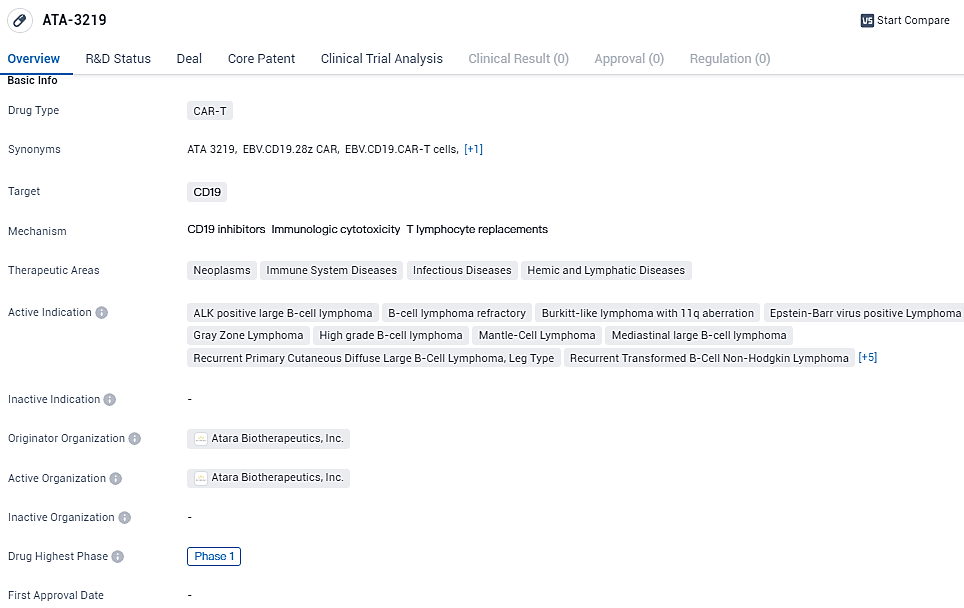
ATA3219 is an innovative allogeneic anti-CD19 CAR T-cell therapeutic approach. It is composed of allogeneic EBV T cells programmed with a CAR that zeroes in on the CD19 antigen, commonly found on the surface of B cells that play a role in the autoimmune response. ATA3219 stands out due to proprietary technologies resulting in T-cell memory features and sustainability, increased cell growth while avoiding cell weariness through a unique 1XX costimulatory domain, plus the preservation of the innate T-cell receptor, boosting cell longevity.
The use of an allogeneic strategy may overcome the substantial hurdles related to complexity, operational demands, production expenses, and accessibility issues that come with autologous CAR T solutions, thus enabling prompt treatment for a significant number of patients at elevated risk. This method simplifies treatment administration for doctors and patients alike by negating the need for individual apheresis and customized production, as ATA3219 could readily be administered from an available, pre-prepared treatment stock.
"We are notably thrilled about moving this allogeneic CD19 CAR T into clinical trials given its unique features, encompassing several clinically-proven properties," declared Cokey Nguyen, Chief Scientific and Technical Officer at Atara. "Our aspiration is to verify that ATA3219 can induce profound, persistent remission, permitting the immune system to rejuvenate, and ultimately redefine the therapeutic landscape with a ready-to-use CAR T strategy."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
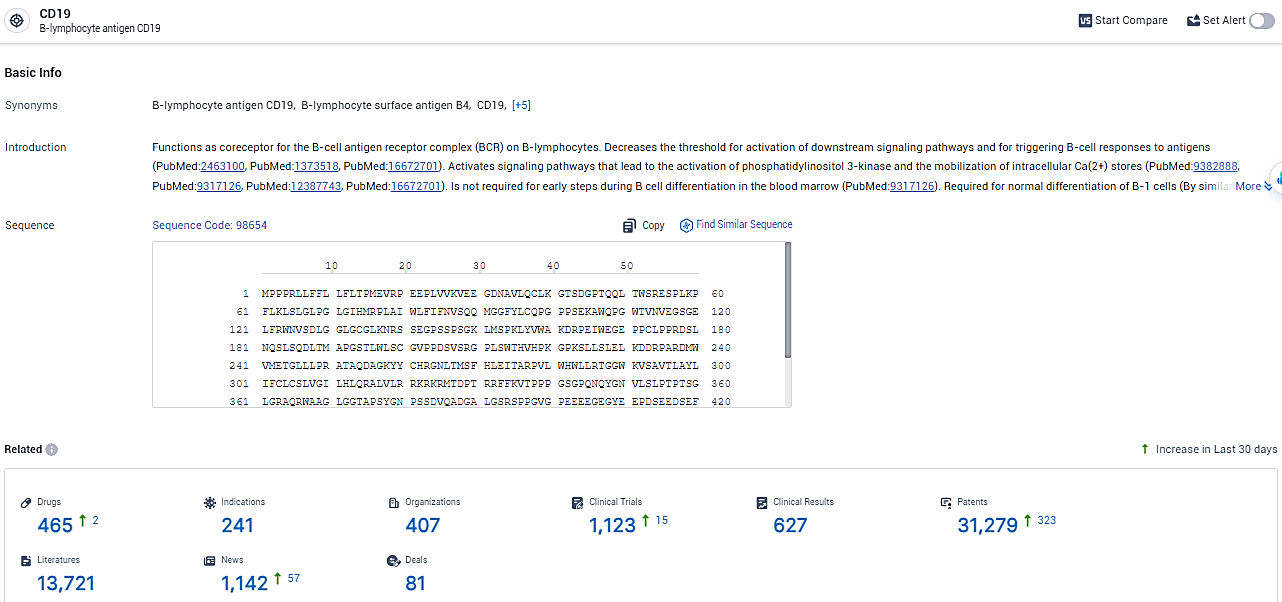
According to the data provided by the Synapse Database, As of February 20, 2024, there are 465 investigational drugs for the CD19 target, including 241 indications, 407 R&D institutions involved, with related clinical trials reaching 1123, and as many as 31279 patents.
ATA-3219 targets CD19 and has potential applications in various therapeutic areas, including neoplasms, immune system diseases, infectious diseases, and hemic and lymphatic diseases. The drug is currently in Phase 1 of clinical development and has shown promise in treating multiple indications related to B-cell lymphomas and other B-cell malignant neoplasms.