Auron Unveils Preliminary Findings on AUTX-703 for AML at ASH Conference
Auron Therapeutics, a biotech firm specializing in creating advanced targeted cancer treatments by targeting cancerous cell states, has released data on its primary project, AUTX-703, at the 66th ASH Annual Meeting and Exposition in San Diego, CA.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
During the ASH conference, Auron Therapeutics showcased AUTX-703's cell line activity, along with ex vivo and in vivo patient-derived xenograft (PDX) data. AUTX-703 is a potent, selective, and orally bioavailable heterobifunctional degrader of KAT2A/B, a histone acetyltransferase linked to AML and other cancers. The treatment was well-tolerated and facilitated cellular differentiation, leading to enhanced survival in a primary patient-derived orthotopic AML model. Auron plans to file several Investigational New Drug (IND) applications for AUTX-703 by the end of 2024, aiming to start clinical development in early 2025.
"AUTX-703's ability to degrade KAT2A/B and promote cellular differentiation, resulting in a significant survival benefit in a primary AML model, is a groundbreaking finding," stated Kate Yen, Ph.D., Founder and CEO of Auron. "These results underscore AURIGIN's platform's effectiveness in pinpointing promising oncology targets for various cancer types as we prepare to submit multiple INDs for AUTX-703 this year."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
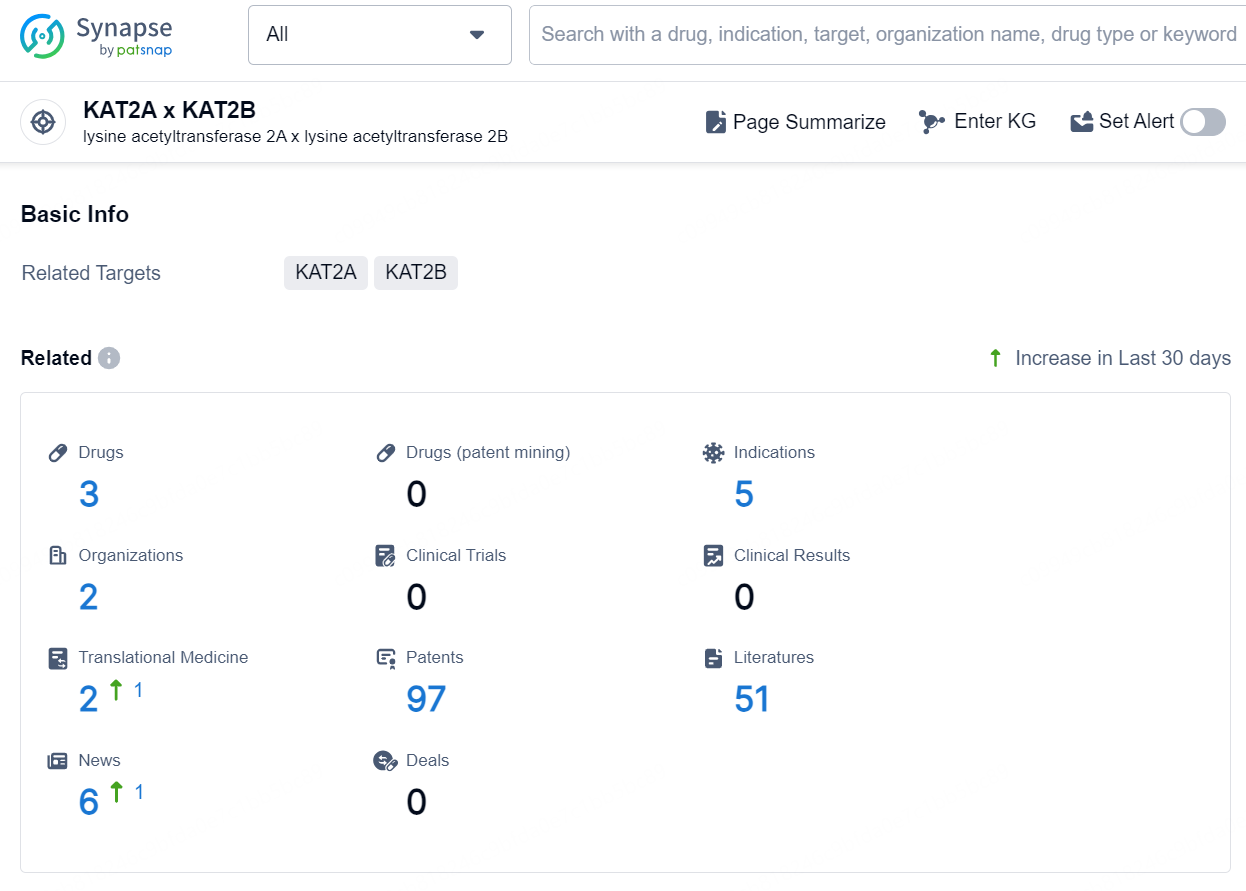
According to the data provided by the Synapse Database, As of December 13, 2024, there are 3 investigational drugs for the KAT2A and KAT2B target, including 5 indications, 2 R&D institutions involved,and as many as 97 patents.
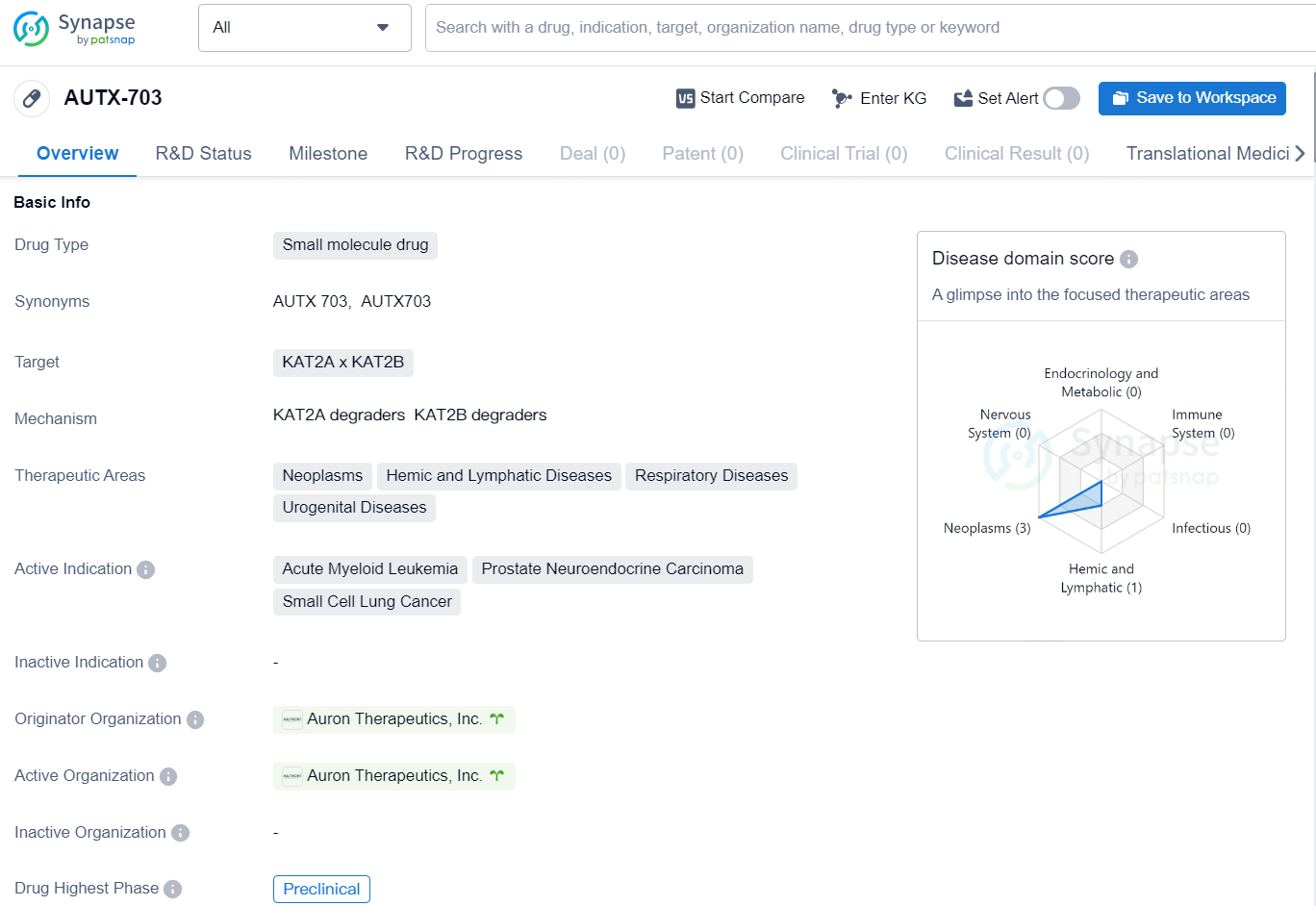
AUTX-703 is a small molecule drug developed by Auron Therapeutics, Inc. The drug targets KAT2A and KAT2B and is currently in the preclinical phase of development. The therapeutic areas for AUTX-703 include neoplasms, hemic and lymphatic diseases, respiratory diseases, and urogenital diseases.