Avirmax Biopharma Launches Clinical Trial for ABI-110 Gene Therapy in Wet AMD and PCV
Avirmax Biopharma, Inc., a pioneer in advancing gene therapy solutions, announced that the initial patient has been successfully administered treatment in the Phase I/IIa clinical trial of ABI-110, the company’s inaugural gene therapy product aimed at addressing Wet Age-related Macular Degeneration (AMD), which encompasses Polypoidal Choroidal Vasculopathy (PCV).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are excited to share this important achievement in the clinical study of ABI-110,” stated Shawn Liu, Ph.D., the CEO of Avirmax Biopharma Inc. “The potential of ABI-110 could transform the treatment options available for Wet AMD and PCV.”
Avirmax has administered the initial dose to a participant in its Phase I/IIa trial for ABI-110, an innovative gene therapy targeting Wet AMD and PCV.
“Dosing the first patient is a crucial advancement in Avirmax’s objective to deliver revolutionary gene therapies to individuals grappling with severe retinal disorders,” commented Wendy Murahashi, M.D., the Chief Medical Officer of Avirmax Biopharma Inc.
Wet Age-related Macular Degeneration (AMD) and Polypoidal Choroidal Vasculopathy (PCV) are serious conditions affecting the retina that can result in significant vision impairment. Existing therapies often necessitate repeated injections and yield only temporary benefits. ABI-110 aims to provide a more durable and effective treatment by targeting the underlying genetic issues associated with these diseases.
ABI-110, the proprietary gene therapy developed by Avirmax Biopharma, employs an engineered capsid, AAV2.N54, to effectively transport the therapeutic transgene to the macular retina. This strategy aspires to deliver a lasting solution that surpasses the constraints of current therapies. The Phase 1 clinical trial is structured to assess ABI-110's safety, tolerability, and initial efficacy in patients suffering from Wet AMD and PCV.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
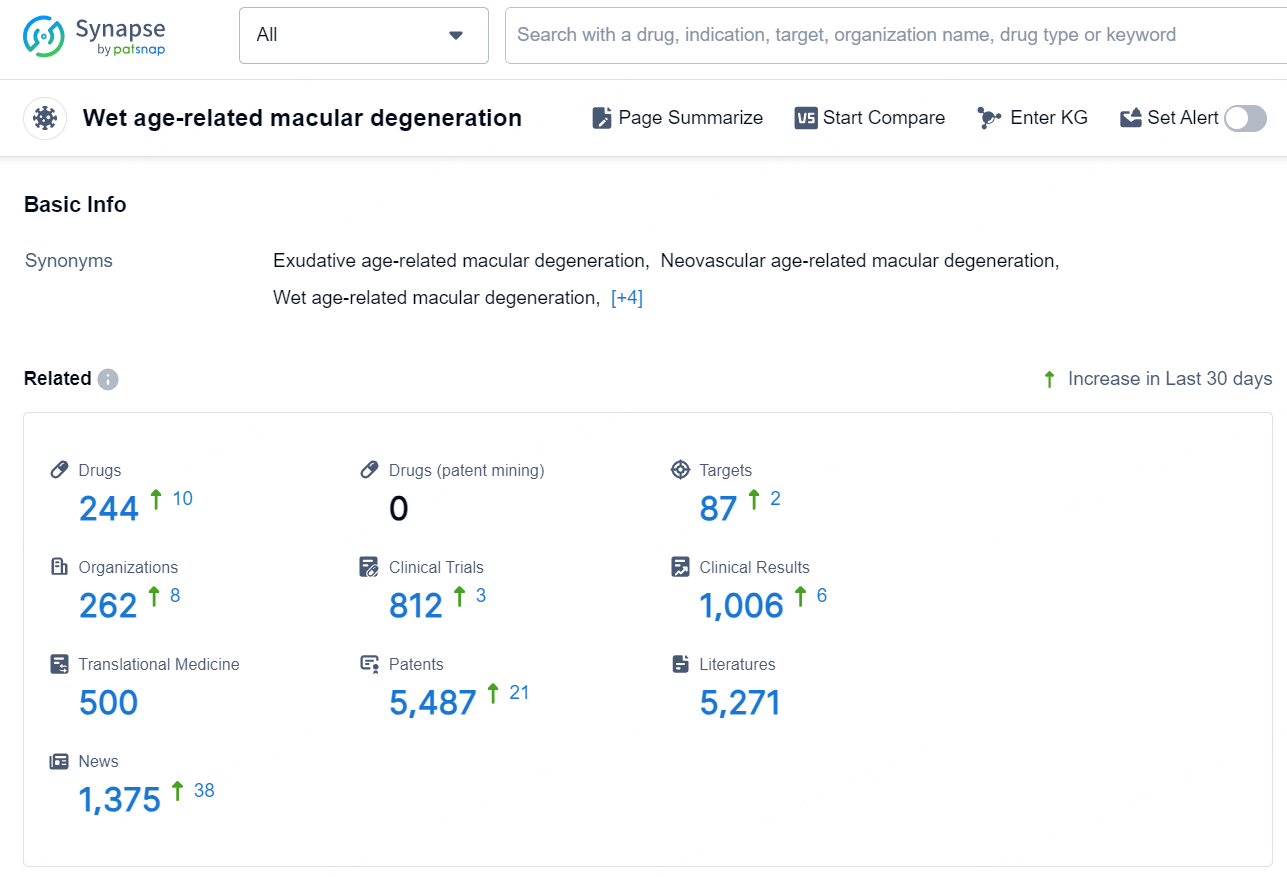
According to the data provided by the Synapse Database, As of December 2, 2024, there are 244 investigational drugs for the wet age-related macular degeneration, including 87 targets, 262 R&D institutions involved, with related clinical trials reaching 812, and as many as 5487 patents..
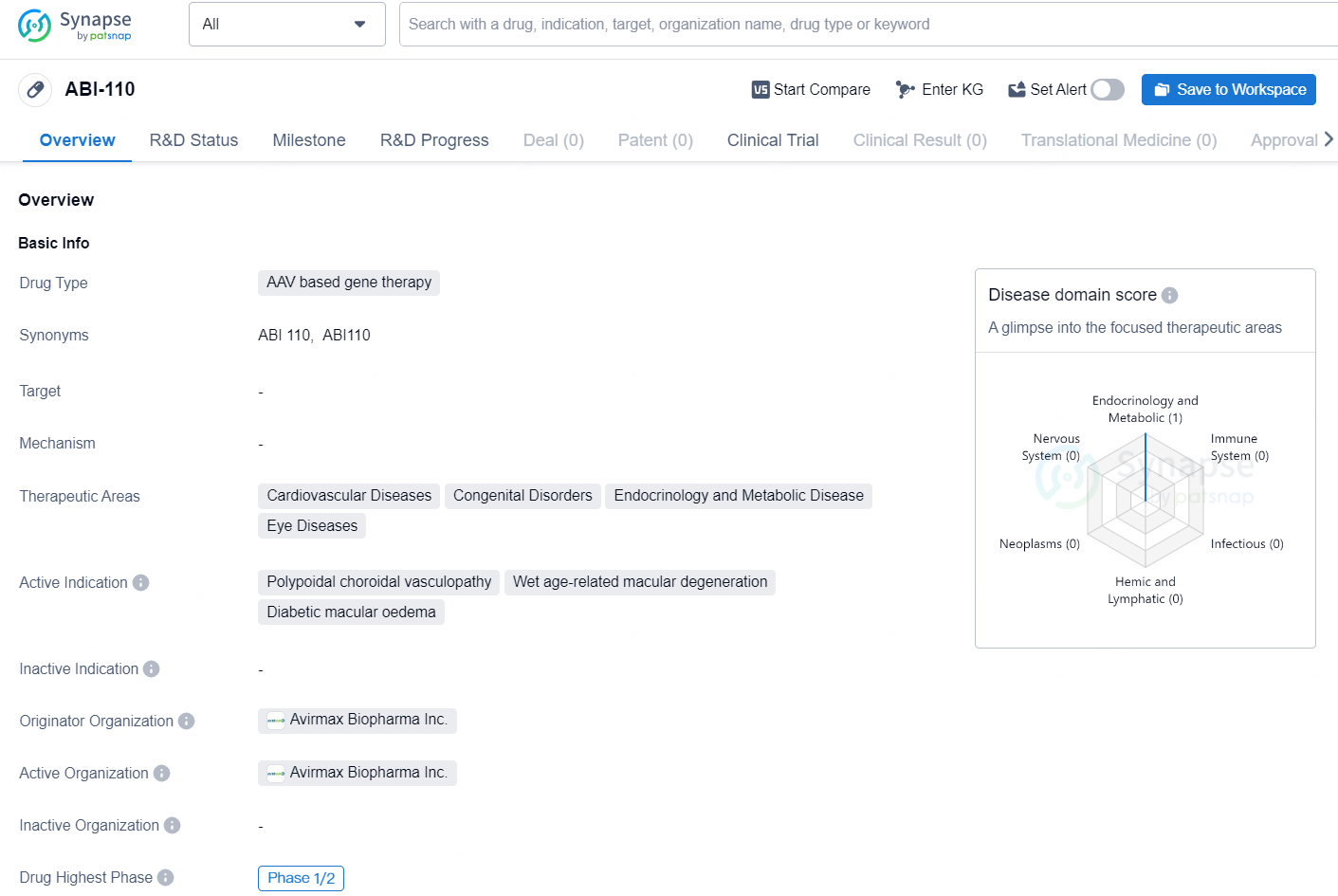
ABI-110 is an AAV based gene therapy drug developed by Avirmax Biopharma Inc. The drug is intended to target several therapeutic areas including cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, and eye diseases. The active indications for ABI-110 are polypoidal choroidal vasculopathy, wet age-related macular degeneration, and diabetic macular oedema.