Bayer Initiates Stage III Assessment for Non-Small Cell Lung Cancer (NSCLC) Treatment
Bayer has disclosed the enrollment of the inaugural patient into the international Phase III SOHO-02 study, a randomized, open-label, multicenter clinical endeavor. This trial aims to evaluate the therapeutic effectiveness and safety profile of the experimental drug BAY 2927088 as a frontline treatment option for patients diagnosed with advanced non-small cell lung cancer (NSCLC) whose tumors harbor activating HER2 mutations.
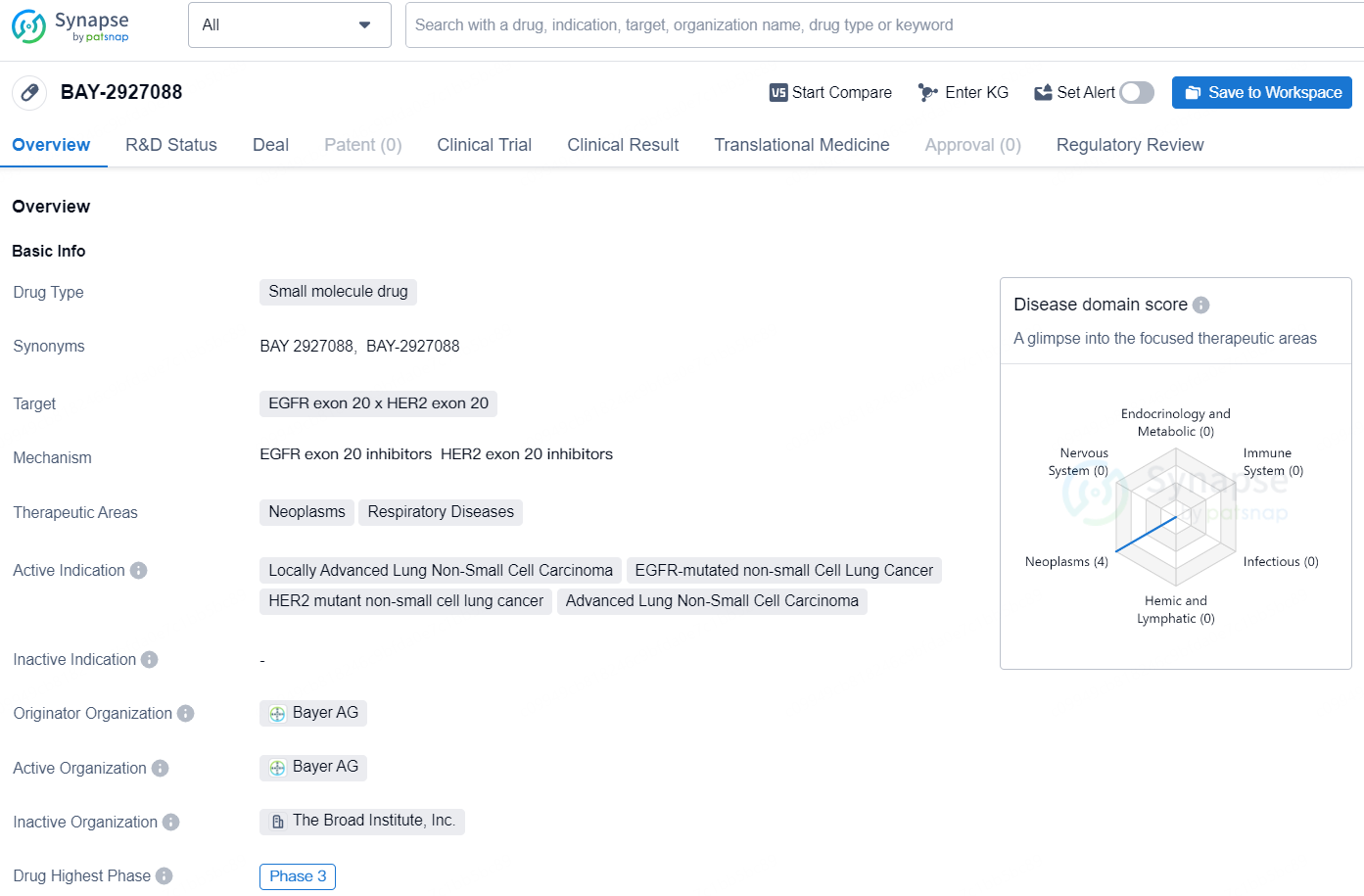
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Apart from the SOHO-02 study, BAY 2927088, an investigational drug, is undergoing evaluation for its potential as a subsequent treatment line for adult patients with unresectable or metastatic NSCLC characterized by activating HER2 (ERBB2) mutations, who have undergone prior systemic therapy. Pivotal findings from the phase I/II SOHO-01 trial will be highlighted during the prestigious presidential symposium at the World Conference on Lung Cancer (WCLC) in San Diego on September 9th, 2024.
"Our dedication to precision medicine transcends mere promises; it is a solemn undertaking to tackle the pressing unaddressed needs of patients battling HER2-mutant NSCLC, a form of the most common lung cancer," affirmed Christian Rommel, Ph.D., Head of Research and Development, Bayer Pharmaceuticals Division. "By propelling groundbreaking research, we strive to enhance survival prospects for those afflicted by this devastating illness. This endeavor exemplifies our steadfast commitment to spearheading precise and tailored healthcare solutions for those in dire need."
BAY 2927088 stems from Bayer's strategic research collaboration with the Broad Institute of MIT and Harvard, situated in Cambridge, MA, USA.
Lung cancer stands as the primary cause of cancer-related mortalities globally. Currently, there exists no approved first-line targeted therapies for NSCLC patients with HER2 activating mutations.
In February 2024, the U.S. Food and Drug Administration (FDA) granted BAY 2927088 Breakthrough Therapy designation for use in the second-line setting. Furthermore, in June 2024, the Center for Drug Evaluation (CDE) in China also awarded BAY 2927088 Breakthrough Therapy status for the same patient demographic.
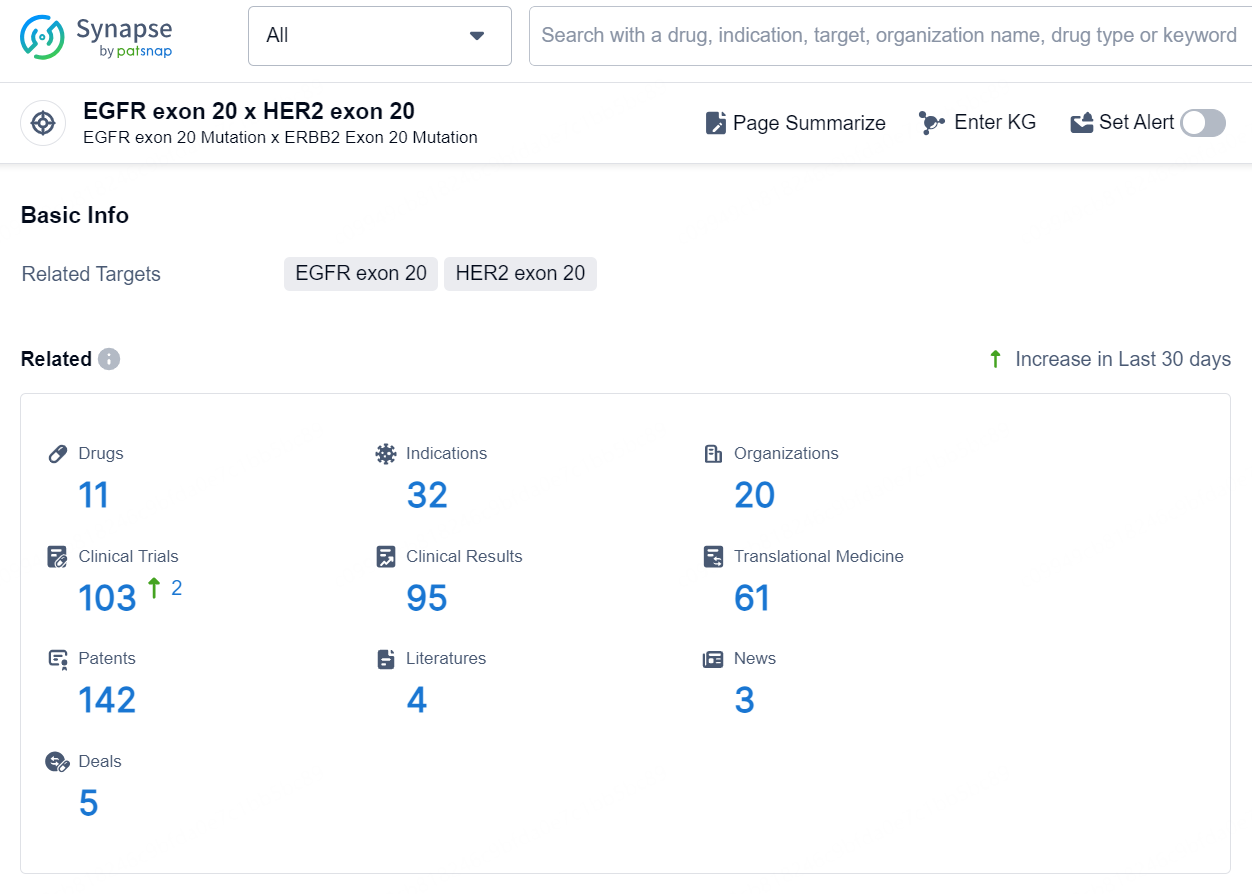
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 2, 2024, there are 11 investigational drugs for the EGFR exon 20 x HER2 exon 20 targets, including 32 indications, 20 R&D institutions involved, with related clinical trials reaching 103, and as many as 142 patents.
BAY-2927088 is a small molecule drug developed by Bayer AG, targeting EGFR exon 20 x HER2 exon 20. The drug is being developed for the treatment of neoplasms and respiratory diseases, with active indications including Locally Advanced Lung Non-Small Cell Carcinoma, EGFR-mutated non-small Cell Lung Cancer, HER2 mutant non-small cell lung cancer, and Advanced Lung Non-Small Cell Carcinoma. Currently, BAY-2927088 has reached its highest phase of development, with Phase 3 trials underway both globally and in China. It is important to note that the drug has been designated as a Breakthrough Therapy, indicating its potential to address unmet medical needs for serious or life-threatening conditions.