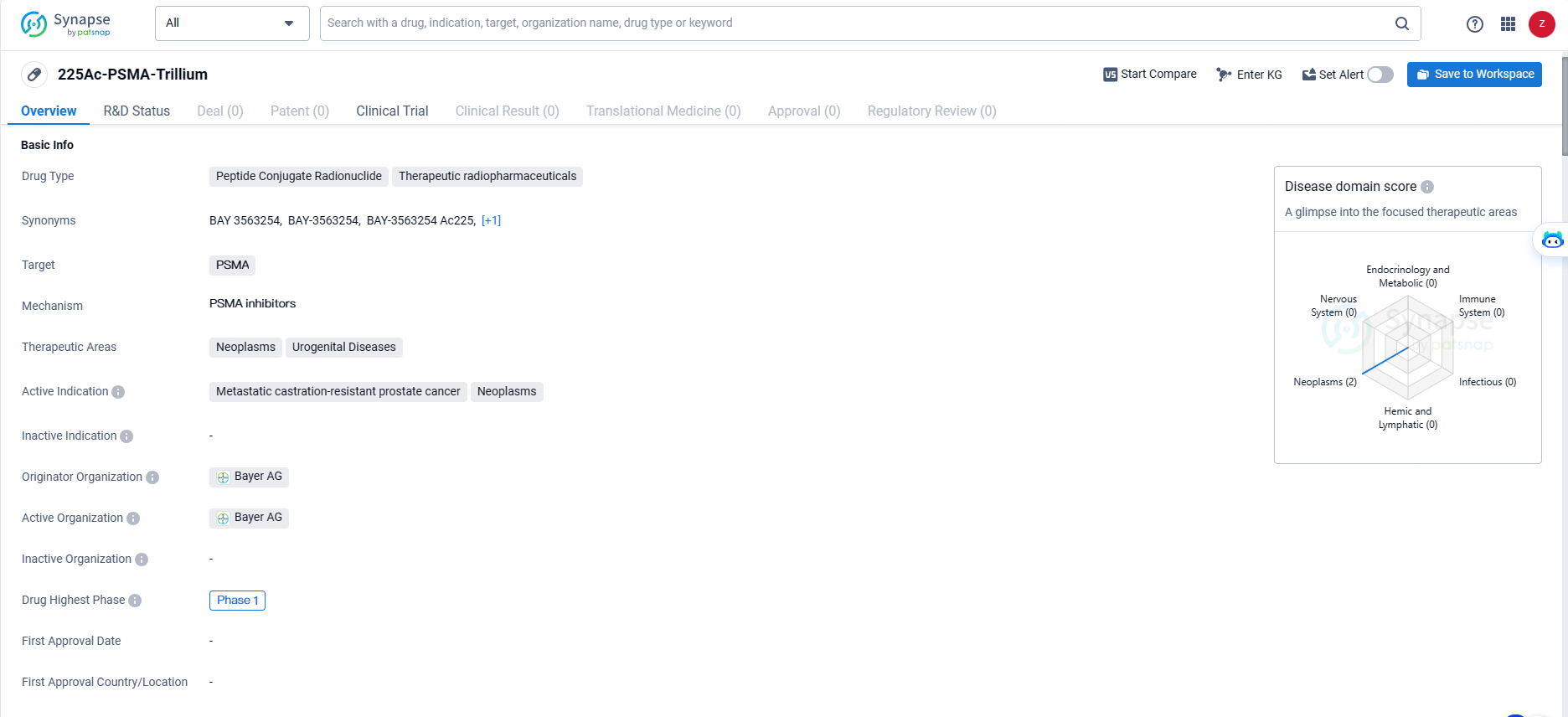
Bayer Starts Phase I Trial of 225Ac-PSMA Targeted Therapy for Advanced Prostate Cancer
Bayer has initiated dosing in a Phase I first-in-human clinical trial for 225Ac-PSMA-Trillium (BAY 3563254), an advanced targeted alpha therapy. This investigational drug, which includes actinium-225 and a new PSMA (prostate-specific membrane antigen) -targeting small molecule with a tailored albumin-binding component, aims to potentially enhance therapeutic effectiveness and minimize adverse effects on normal tissues such as salivary glands.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The dose-escalation research is intended to assess the safety, tolerability, and effectiveness of 225Ac-PSMA-Trillium in individuals suffering from advanced metastatic castration-resistant prostate cancer (mCRPC).
"Though the treatment landscape for prostate cancer has seen recent improvements, there is still a substantial unmet need for innovative precision therapies to better outcomes for mCRPC patients," stated Fred Saad, MD, FRCS, Professor and Head of Surgery at the University of Montreal and Director of Genitourinary Oncology at the University of Montreal Hospital Center (CHUM), Canada. "225Ac-PSMA-Trillium represents a fresh strategy, offering a potential new solution to satisfy this unmet need in mCRPC."
"Targeted radionuclide therapy is a key component of precision oncology at Bayer, with the potential to transform the treatment paradigm for patients whose conditions have become resistant to other therapies," said Dominik Ruettinger, M.D., Ph.D., Head of Research and Early Development for Oncology at Bayer. "We are thrilled to start the phase I trial and dose the first patient with 225Ac-PSMA-Trillium. Given its distinctive design, we believe it could provide significant benefits for patients with metastatic prostate cancer, and we eagerly anticipate progressing the program through clinical stages," Ruettinger added.
Prostate cancer is the second most frequently diagnosed cancer in men1 and is a critical focus area at Bayer. Despite notable advancements in the past decade, mCRPC continues to be a fatal disease with an average survival span of around 31 months.2 Bayer remains dedicated to enhancing medical innovations for prostate cancer patients at all stages of the disease.
In April, Bayer presented 225Ac-PSMA-Trillium during the New Drugs on the Horizon session at the AACR (American Association of Cancer Research) Annual Meeting.3 The findings included preclinical in vitro and in vivo characterization, and results from a Phase 0 clinical imaging and dosimetry study conducted in prostate cancer patients.
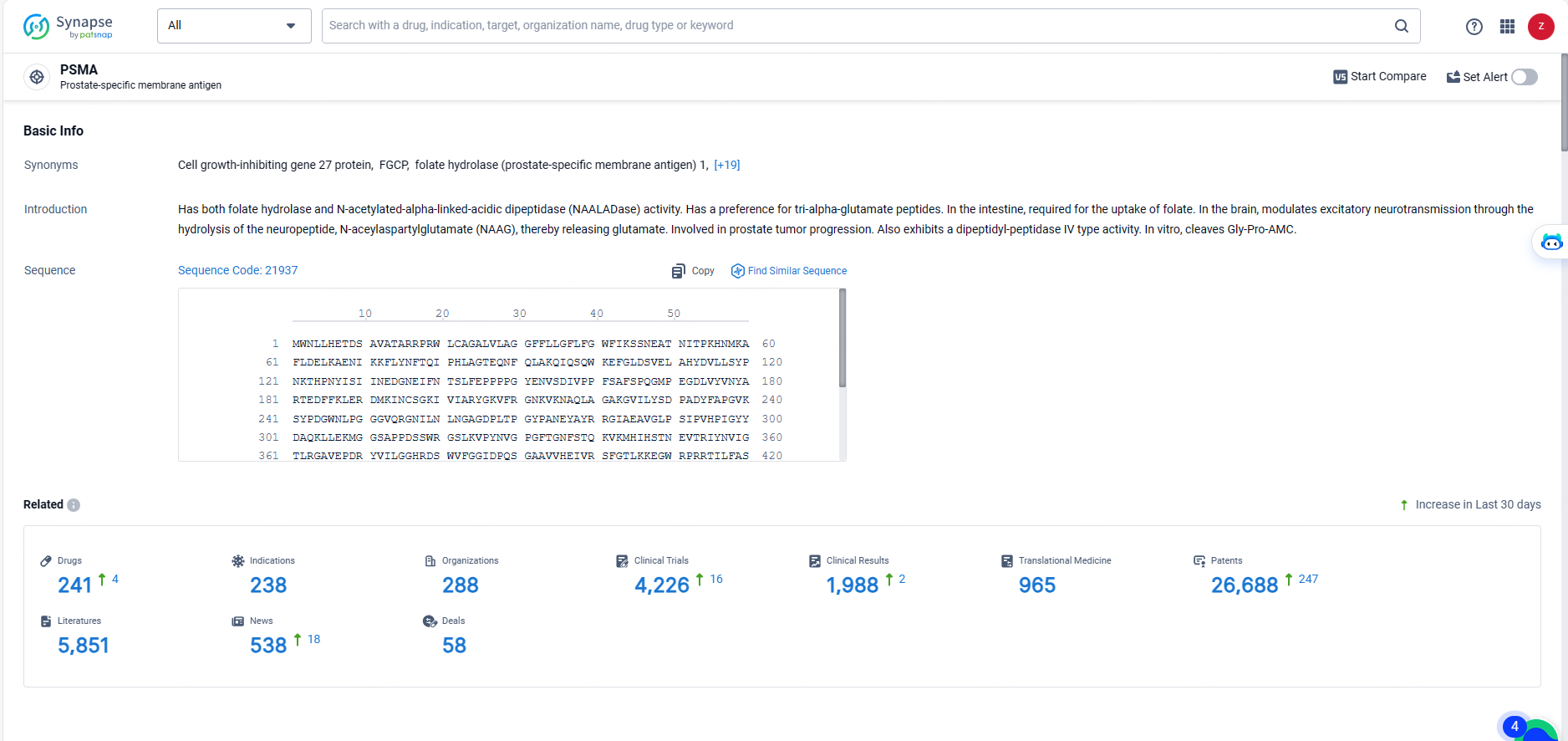
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 241 investigational drugs for the PSMA target, including 238 indications, 288 R&D institutions involved, with related clinical trials reaching 4226, and as many as 26688 patents.
225Ac-PSMA-Trillium represents a significant advancement in the field of biomedicine, particularly in the area of therapeutic radiopharmaceuticals for the treatment of urogenital diseases and neoplasms. As the drug progresses through further clinical trials, it has the potential to offer new treatment options for patients with metastatic castration-resistant prostate cancer and other related cancers.