Biohaven Updates on Taldefgrobep Alfa for SMA and Obesity Development
In the RESILIENT SMA trial, taldefgrobep alpha alpha demonstrated significant enhancements in motor function across all assessment timepoints using the Motor Function Measure-32 scale (MFM-32). However, the treatment group did not achieve statistical distinction from the placebo plus standard of care (SOC) group concerning the primary endpoint at Week 48.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Efficacy signals were identified in clinically significant and biomarker-defined subgroups, including factors such as age, ambulatory capability, existing therapies, and initial myostatin levels.
Analyses of pre-defined subgroups based on race and ethnicity revealed that the predominant study cohort (87% Caucasian; n=180) exhibited significant improvements in the MFM-32 across all assessment points, particularly at Week 48, in comparison to the placebo plus standard of care (SOC) group (p < 0.05). Further examination of these individuals (n=123) with measurable baseline myostatin levels (the pharmacological target for taldefgrobep) indicated enhanced efficacy signals within this myostatin-positive group (p=0.02).
Biohaven intends to communicate with the FDA regarding potential future directions and plans to share the study findings at an upcoming conference. The optional long-term extension of the trial will continue as further data analysis and regulatory discussions proceed.
Prespecified outcome metrics for the overall study population, reflecting changes in body composition at Week 48, indicated a more pronounced reduction in the percentage of total body fat mass in the taldefgrobep group versus the placebo plus SOC group (p=0.008), as assessed by dual energy x-ray absorptiometry (DXA). Additionally, the taldefgrobep group exhibited numerically higher increases in lean muscle mass and bone density compared to the placebo and SOC group.
In light of the consistent and significant effects of taldefgrobep on body composition (i.e., fat mass, lean muscle mass, and bone density), Biohaven aims to expedite the progression of taldefgrobep into a placebo-controlled Phase 2 obesity trial in the fourth quarter of 2024, utilizing an easy-to-use, self-administered autoinjector.
In the RESILIENT study, taldefgrobep showed strong target engagement by reducing myostatin levels below measurable limits in all treated participants over a 48-week period. Furthermore, taldefgrobep was generally well-tolerated in the RESILIENT study, with 97% of participants opting to continue into the optional long-term extension. No serious adverse events (SAEs) related to taldefgrobep treatment were reported.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
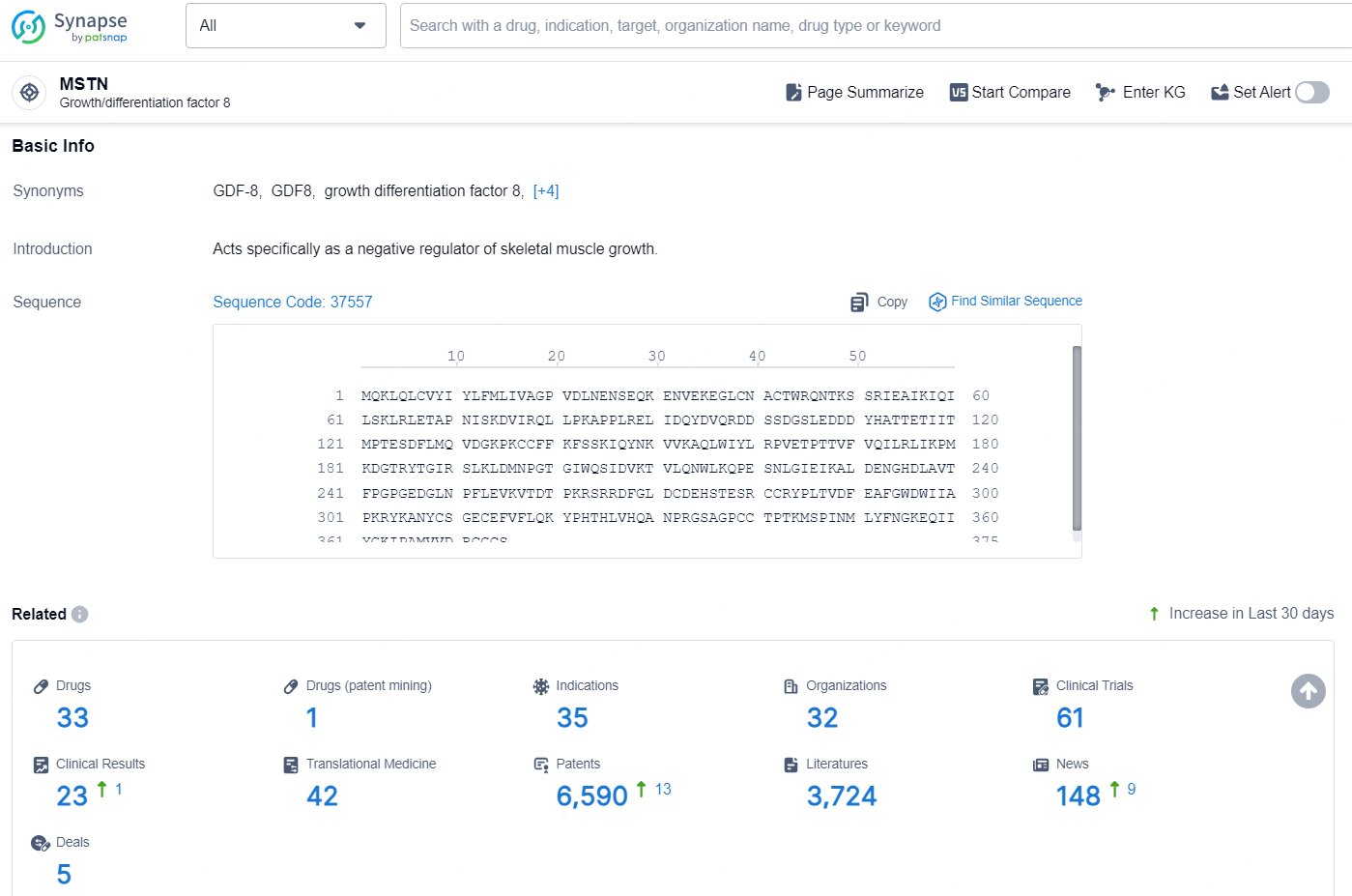
According to the data provided by the Synapse Database, As of December 4, 2024, there are 33 investigational drug for the MSTN targets, including 35 indications, 32 R&D institutions involved, with related clinical trials reaching 61, and as many as 6590 patents.
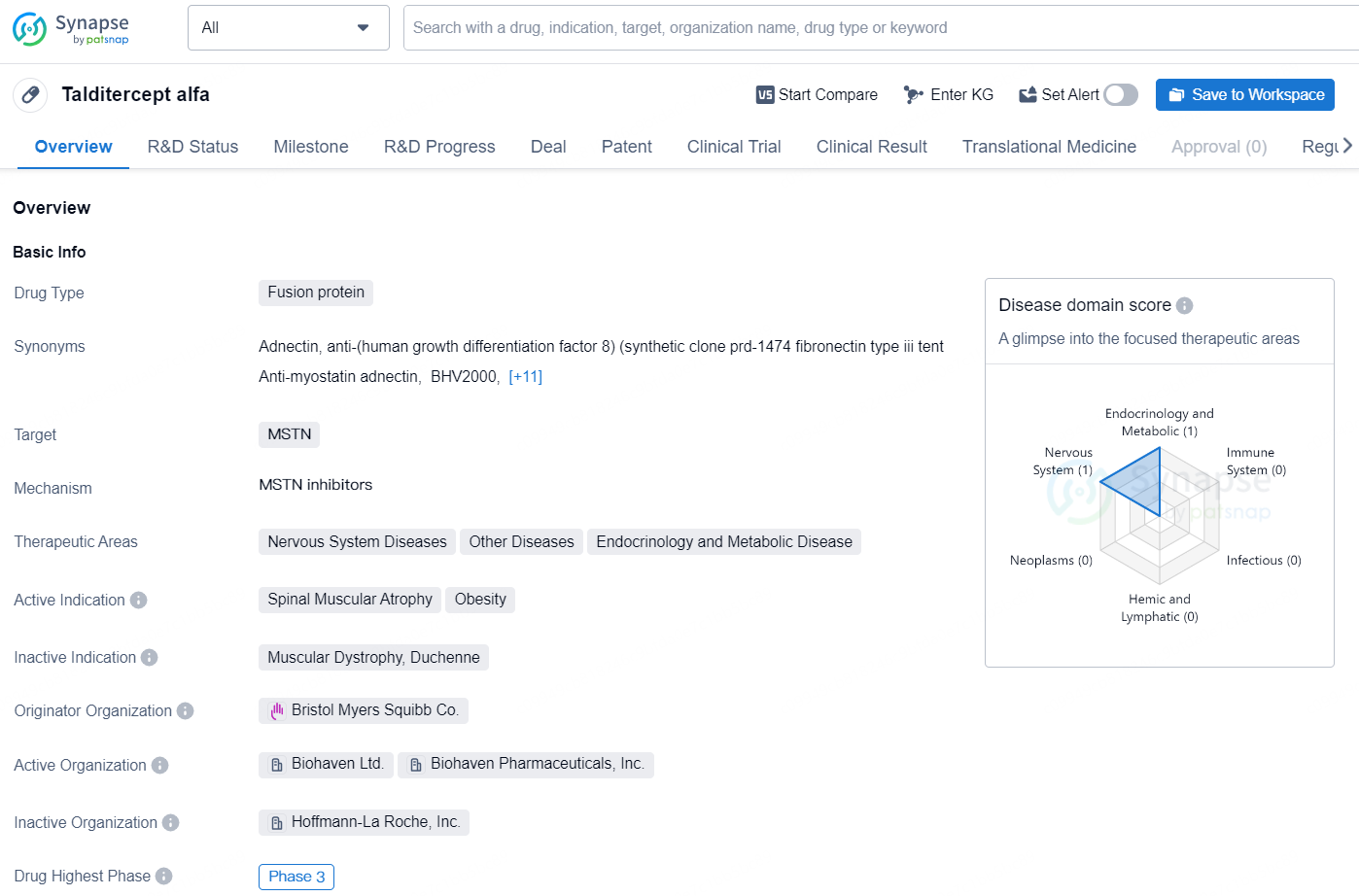
Talditercept alfa is a fusion protein drug developed by Bristol Myers Squibb Co. The drug targets myostatin (MSTN) and falls within the therapeutic areas of Nervous System Diseases, Other Diseases, and Endocrinology and Metabolic Disease. Talditercept alfa is currently in its highest global phase of development, specifically Phase 3, indicating that it has progressed through earlier stages of clinical trials and is now undergoing advanced testing to assess its safety and efficacy.