BioPharma Accord, Inc. announces FDA approval for their STELARA® biosimilar, DMB-3115 license application
The American branch specializing in pharmaceuticals under Intas Pharmaceuticals Ltd., known as Accord BioPharma, Inc., which concentrates on the advancement of treatments in the fields of cancer, immune system disorders, and intensive care medicine, has made a public announcement that the United States regulatory authority, the FDA, has approved their request for a license, specifically the Biologics License Application, for the product named DMB-3115.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
DMB-3115 is being developed as an equivalent alternative to the widely prescribed drug STELARA®, created by Janssen Biotech, Inc. This drug has achieved widespread approval for managing various ailments, including psoriatic skin conditions, joint inflammation tied to psoriasis, Crohn's disease, and ulcerative colitis. In the U.S. market, STELARA's sales reached an impressive $13.9 billion, affirming its position as one of the top-selling therapeutic biological products.
"The established effectiveness of STELARA in combating auto-immune disorders is noteworthy, and we’re enthusiastic about forging paths that allow greater patient access to this much-needed therapy for such burdensome health conditions," stated Chrys Kokino, who heads Accord in the U.S. "Part of our commitment to advancing healthcare lies in mitigating the economic strain on patients and the American healthcare infrastructure by introducing cost-efficient options like biosimilars."
A collaborative venture for developing DMB-3115 began in 2013, led by Dong-A Socio Holdings and Meiji Seika Pharma. Intas Pharmaceuticals secured exclusive rights to market the product after entering an alliance unveiled in 2021. Accord BioPharma, an Intas Pharmaceuticals affiliate, will handle market introduction in the United States.
"This collaboration with Dong-A Socio Holdings and Meiji Seika Pharma exemplifies our enduring dedication to enhance worldwide patient access to premium biosimilar therapies," expressed Mr. Binish Chudgar, the vice chairman and managing director of Intas Pharmaceuticals.
The application for marketing authorization of DMB-3115 hinges on data from stage III global clinical trials involving individuals with plaque psoriasis. The principal measure was the change rate in the Psoriasis Area and Severity Index, focusing on skin-related symptoms. These clinical evaluations indicated a high degree of similarity between DMB-3115 and the originator biologic, ustekinumab, with no significant differences in quality, safety, or therapeutic effectiveness.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
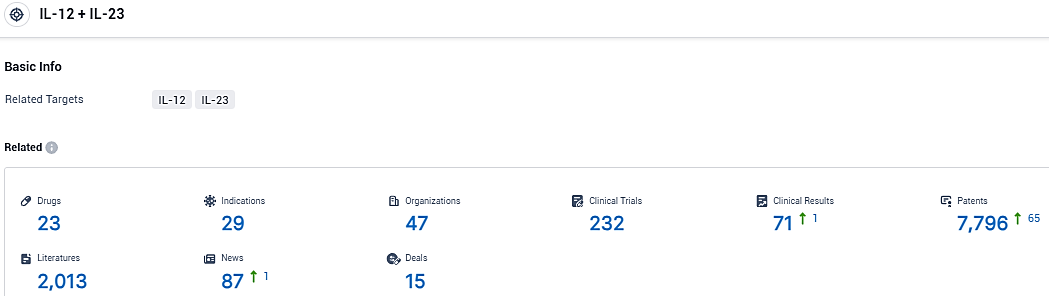
According to the data provided by the Synapse Database, As of January 10, 2024, there are 23 investigational drugs for the IL-12 and IL-23 target, including 29 indications, 47 R&D institutions involved, with related clinical trials reaching 232, and as many as 7796 patents.
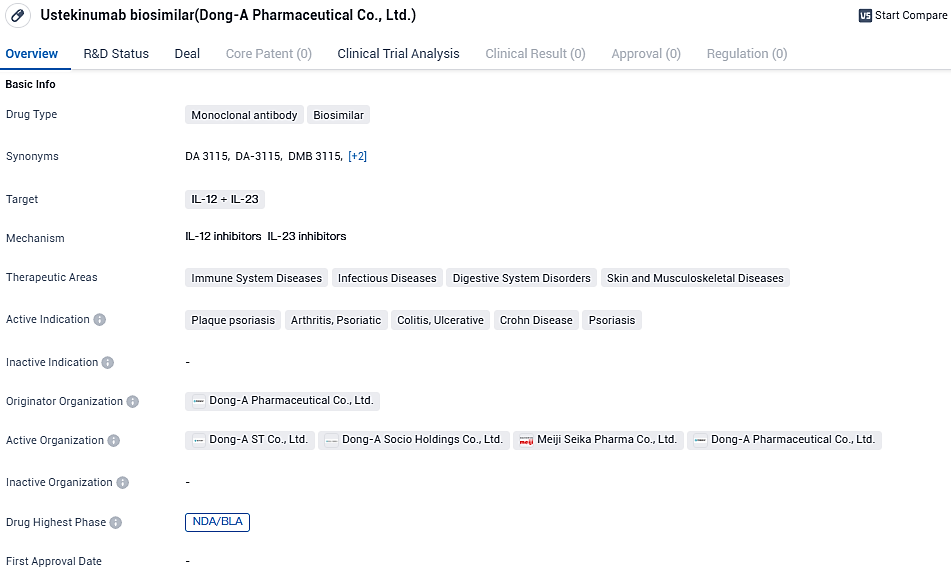
Ustekinumab biosimilar targets IL-12 and IL-23. It has shown potential in treating immune system diseases, infectious diseases, digestive system disorders, as well as skin and musculoskeletal diseases. The active indications for this drug include plaque psoriasis, psoriatic arthritis, colitis ulcerative, Crohn's disease, and psoriasis. As the highest phase achieved globally is NDA/BLA, it indicates that the drug has undergone rigorous testing and evaluation, positioning it for potential regulatory approval.