BMS reports improved survival in operable non-small cell lung cancer patients with a regimen of Opdivo (nivolumab) and chemotherapy
Bristol Myers Squibb declared that the Phase 3 CheckMate -77T study achieved its main objective of enhanced event-free survival, as evaluated by the Blinded Independent Central Review, in patients suffering from resectable stage IIA to IIIB non-small cell lung cancer.
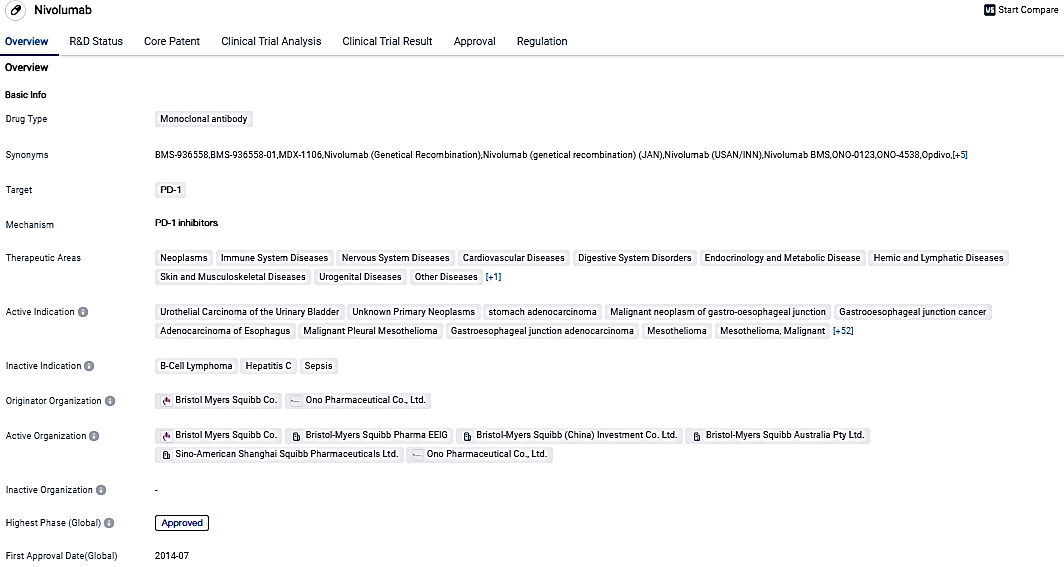
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In a preplanned interim assessment, the perioperative treatment combination of neo adjuvant Opdivo(nivolumab) and chemotherapy, followed by a surgical intervention and then adjuvant Opdivo, demonstrated a statistically significant and clinically relevant enhancement in EFS opposed to neoadjuvant chemotherapy and placebo, which is subsequently followed by surgery and adjuvant placebo. The safety attributes of this Opdivo-centric regimen aligned with prior studies conducted on NSCLC.
Remarkable scientific progress has been witnessed in treating non-metastatic non-small cell lung cancer, and our commitment stays strong in discovering innovative approaches that can assist more patients in achieving superior long-term results," stated Abderrahim Oukessou, M.D., the global program leader of thoracic cancers and the vice president of Bristol Myers Squibb.
The company is looking to conduct a thorough evaluation of the data generated from the CheckMate -77T and is eager to present the findings to the scientific fraternity via a future medical conference. It also plans to engage in discussions with health authorities. The trial is presently underway to determine the overall survival, which is a secondary endpoint.
Opdivo is an immune checkpoint inhibitor of programmed death-1 (PD-1), developed to harness the body's own immune system in an unique way for the restoration of anti-tumor immune response. Opdivo, and combinations based on it, to this day, demonstrated an enhanced efficacy in the neoadjuvant, adjuvant or perioperative management of four types of tumors: lung cancer, bladder cancer, esophageal/gastroesophageal junction cancer and melanoma.
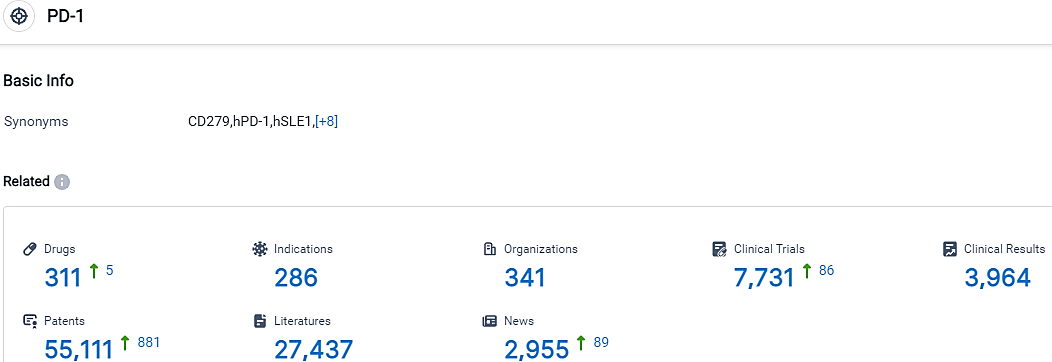
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 311 investigational drugs for the PD-1 target, including 286 indications,341 R&D institutions involved, with related clinical trials reaching 7731,and as many as 55111 patents.
Nivolumab, a monoclonal antibody medication, is expertly designed to aim at PD-1 and displays encouraging outcomes in the treatment of numerous cancer forms. Its broad application across diverse therapeutic zones, combined with its various regulatory approvals, signifies its prospective value in the domain of biomedicine progress.