Boehringer gains U.S. FDA Breakthrough Therapy status and starts two phase III MASH trials for survodutide
Boehringer Ingelheim has revealed that survodutide (BI 456906), a dual glucagon/GLP-1 receptor agonist intended for treating adults with non-cirrhotic metabolic dysfunction-associated steatohepatitis (MASH) and moderate to advanced fibrosis (stages 2 or 3), has received the Breakthrough Therapy designation from the U.S. Food and Drug Administration (FDA). This designation is intended to accelerate the development and evaluation of drugs for severe or life-threatening conditions that have shown early clinical evidence of significant improvement compared to current treatment options.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Boehringer recently revealed the start of two Phase III clinical studies for survodutide aimed at treating adults with MASH and fibrosis. The LIVERAGE trial will investigate if survodutide can enhance conditions of MASH and/or fibrosis over a 52-week period and lower the chances of progressing to end-stage liver disease after around seven years for individuals with moderate or severe liver fibrosis (stages 2 or 3). Meanwhile, LIVERAGE-Cirrhosis will assess whether survodutide can decrease the risk of end-stage liver disease outcomes after about four and a half years in patients with MASH and compensated cirrhosis (fibrosis stage 4), where extensive liver scarring occurs.
Dr. Arun Sanyal, M.D., a Professor of Medicine at Virginia Commonwealth University School of Medicine, emphasized, “Given the heavy impact of MASH and the scarcity of therapeutic options, it is crucial to explore new treatments. The Phase III LIVERAGE studies offer a promising chance to evaluate whether survodutide, utilizing its dual action on glucagon and GLP-1 receptors, can meet this critical healthcare need.”
Shashank Deshpande, Boehringer Ingelheim's Head of Human Pharma, stated, “As the number of MASH cases is projected to increase globally, it’s essential we deepen our understanding of this ailment.” The survodutide Phase III trial initiative is among the largest worldwide, in terms of the number of countries and sites included. Intriguingly, the novel design aims specifically at advanced fibrosis, such as cirrhosis due to MASH, which has the greatest need for treatment, potentially transforming the future of therapy. The Breakthrough Therapy designation highlights that this potentially leading therapy could revolutionize MASH treatment.
Survodutide is licensed to Boehringer Ingelheim from Zealand Pharma, with Boehringer taking exclusive charge of its development and distribution internationally. Zealand retains co-promotion rights within the Nordic region.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
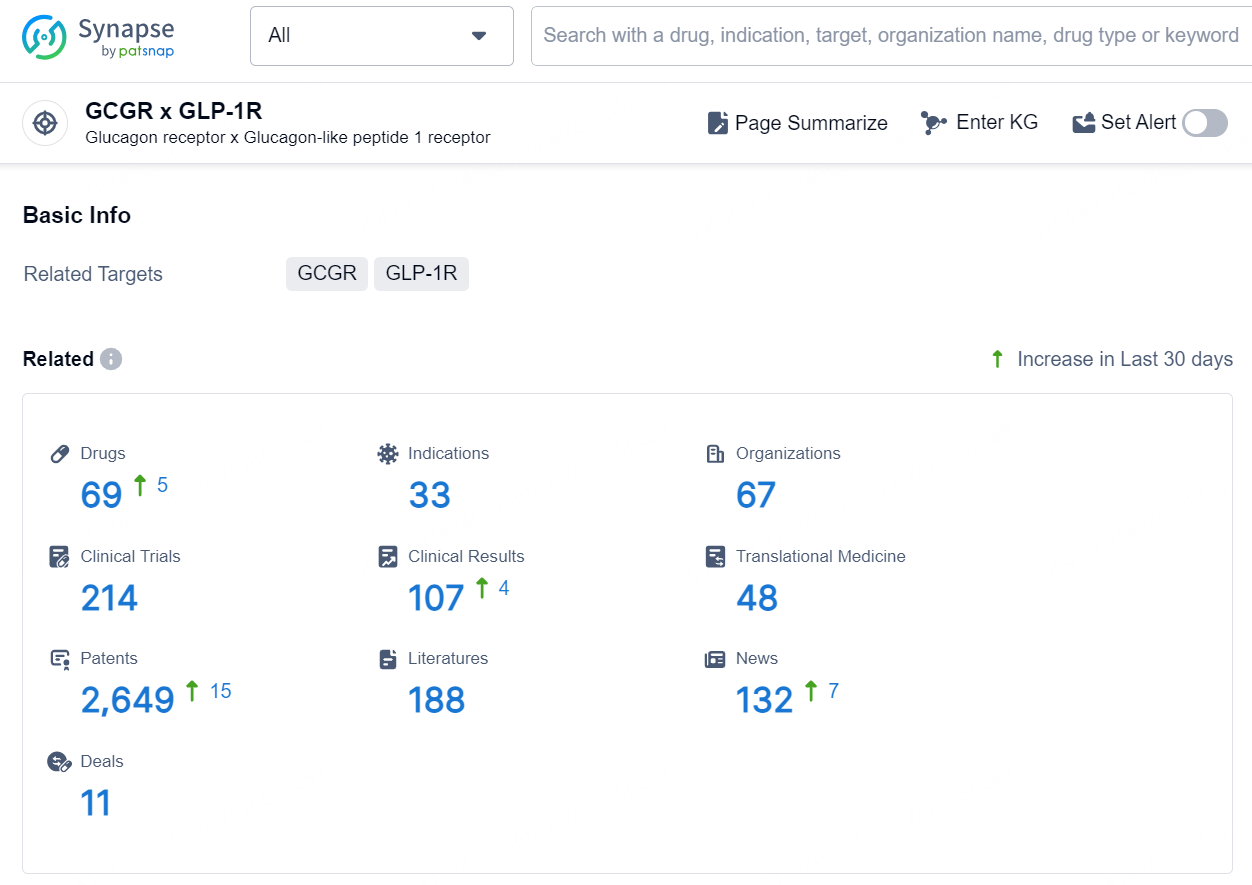
According to the data provided by the Synapse Database, As of October 10, 2024, there are 69 investigational drug for the GCGR x GLP-1R target, including 33 indications, 67 R&D institutions involved, with related clinical trials reaching 214, and as many as 2649 patents.
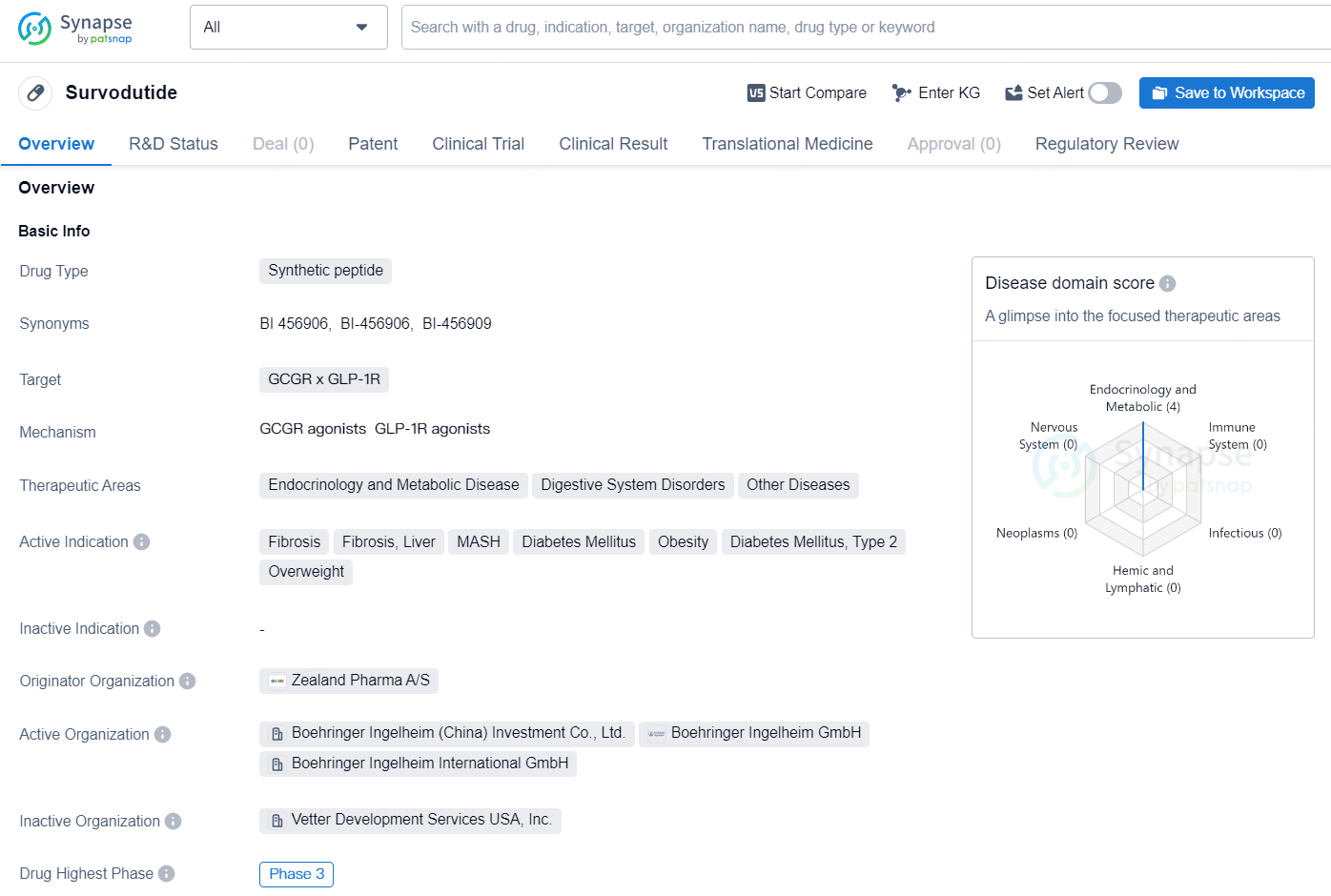
Survodutide is a synthetic peptide drug that targets the GCGR x GLP-1R receptors and is being developed by Zealand Pharma A/S. The drug falls within the therapeutic areas of Endocrinology and Metabolic Disease, Digestive System Disorders, and Other Diseases. Survodutide is being developed for the treatment of fibrosis, liver fibrosis, MASH, diabetes mellitus, obesity, diabetes mellitus type 2, and overweight.