Boston Pharma Presents Data at EASL 2024: BOS-580 Improves Lipid Profiles in MASH Patients
Boston Pharmaceuticals, a company focused on clinical-stage biopharmaceuticals, is working on creating unique molecules to treat critical liver conditions. Recently, they released new data from a Phase 2a study, showing that BOS-580—a research-stage, long-acting analogue of fibroblast growth factor 21 (FGF21)—has favorable effects on the circulating lipidome. Additionally, this investigational drug influences changes in the metabolomics-based advanced steatohepatitis fibrosis score among patients suffering from phenotypic metabolic dysfunction-associated steatohepatitis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Our research revealed that the circulating lipidome in phenotypic MASH patients is different from that observed in obese but otherwise healthy adults. Administering BOS-580 to these patients over a 12-week period significantly enhanced their lipid profiles and lowered MASEF scores, a new composite biomarker designed to identify at-risk MASH patients," stated Sophie Kornowski, CEO of Boston Pharmaceuticals.
"The evidence continues to underscore BOS-580’s promise as a potent once-monthly therapy for MASH. Additionally, the findings support the importance and prospective application of diagnostic and predictive biomarkers in MASH for evaluating and managing patients. We anticipate presenting biopsy outcomes from our current study in patients with stage F2/F3 fibrosis due to MASH later this year," Sophie Kornowski further commented.
Alterations in the lipidome observed in MASH patients imply that disruptions in lipid metabolism might be essential to the disease’s onset, contributing to oxidative stress, inflammation, and cellular damage. Gaining insights into the role of lipid metabolism in both healthy and diseased states could propel the identification of potential diagnostic biomarkers and new therapeutic targets.
The MASEF score, driven by metabolomics, provides a noninvasive method to recognize patients with at-risk MASH who could gain from medical treatments and intervention, highlighting its potential as a valuable diagnostic tool.
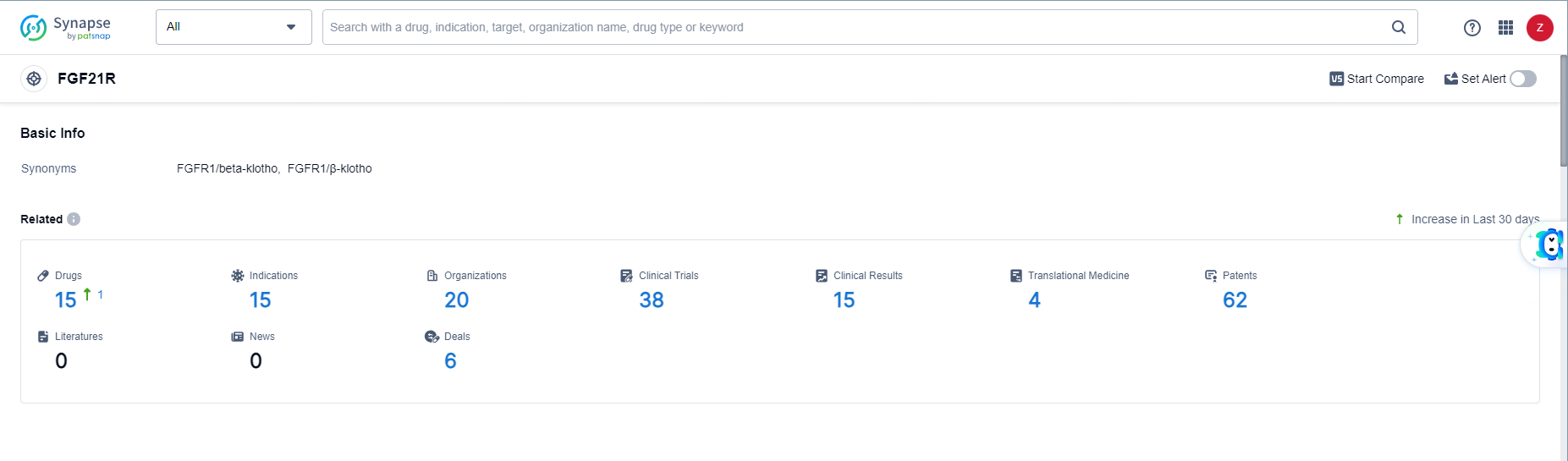
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 11, 2024, there are 15 investigational drugs for the FGF21R targets, including 15 indications, 20 R&D institutions involved, with related clinical trials reaching 38, and as many as 62 patents.
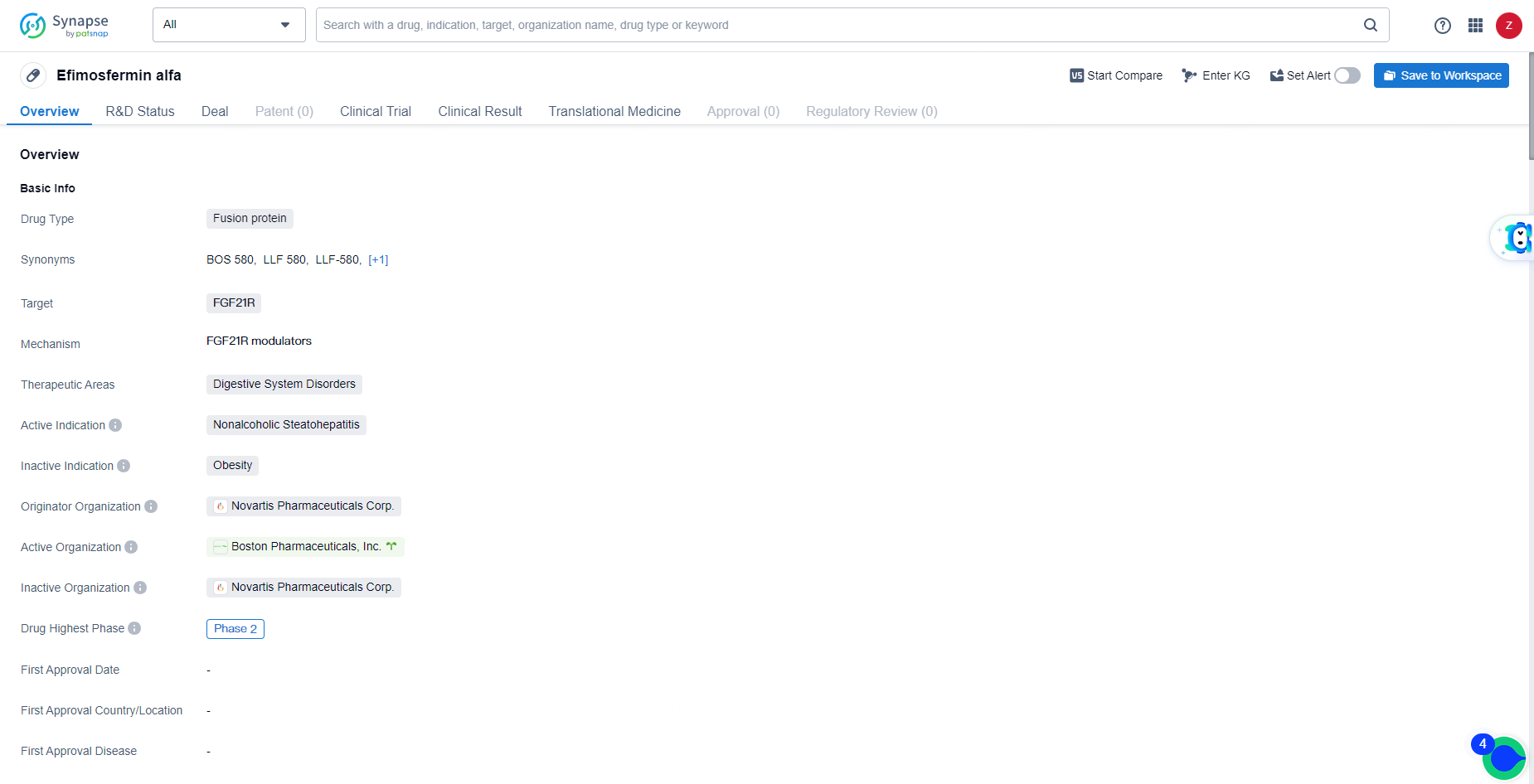
Efimosfermin alfa represents a potential advancement in the field of biomedicine, particularly in the treatment of digestive system disorders such as nonalcoholic steatohepatitis. As the drug progresses through clinical development, further data and insights will be needed to assess its potential impact on patient outcomes and its eventual market potential.