BRG01 Enters Phase II: Biosyngen's Autologous EBV-Specific CAR-T Therapy for Advanced Nasopharyngeal Cancer
Biosyngen, a premier biotechnology firm specializing in pioneering cell therapies, has recently revealed that China's Center for Drug Evaluation of the National Medical Products Administration has granted approval for the commencement of a crucial Phase II clinical trial. This trial will assess BRG01, the company's autologous Epstein-Barr virus (EBV) specific chimeric antigen receptor T-cell therapy, in patients suffering from recurrent or metastatic EBV-positive nasopharyngeal carcinoma.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 BRG01 has achieved a groundbreaking milestone as the first CAR-T therapy for solid tumors to secure clinical trial approvals in both China and the U.S., and to advance to a Phase II pivotal clinical trial. This marks a significant advancement in the domain of cell-based immunotherapies targeting solid malignancies.
BRG01 has achieved a groundbreaking milestone as the first CAR-T therapy for solid tumors to secure clinical trial approvals in both China and the U.S., and to advance to a Phase II pivotal clinical trial. This marks a significant advancement in the domain of cell-based immunotherapies targeting solid malignancies.
“The Phase II clinical trial approval for BRG01 underscores the solid preclinical data and promising early clinical outcomes seen with this novel treatment,” stated Professor Zhang Li, Director of the Phase I Ward at Sun Yat-Sen University Cancer Center and Deputy Director of the Lung Cancer Research Institute at Sun Yat-Sen University, who is the lead investigator for the BRG01 trials. “BRG01 has the potential to be a pioneering T-cell therapy for EBV-positive tumors, and we are optimistic about its capacity to provide substantial clinical benefits to patients with this challenging cancer.”
Patient enrollment for the Phase I clinical trial of BRG01 in China and the U.S. concluded in January of this year, with all participants receiving BRG01 infusions as part of the registered trial. This Phase I trial successfully addressed dose-limiting toxicity and preliminary efficacy in nine patients with advanced nasopharyngeal carcinoma, all of whom had undergone treatment with at least one immune checkpoint inhibitor, such as a PD-1 antibody.
Preliminary data from this study revealed outstanding safety and promising clinical activity, evidenced by 75% of high-dose patients experiencing local tumor reduction and decreased metabolic activity on PET-CT scans, with some patients achieving complete remission. Additionally, BRG01 demonstrated significant anti-EBV efficacy, with notable reductions in peripheral blood EBV viral load post-treatment.
These results underscore BRG01’s potential in cancer therapy and its dual antiviral benefits, creating a strong foundation for future clinical applications. These findings were crucial for the CDE's decision to move BRG01 to Phase II clinical trials.
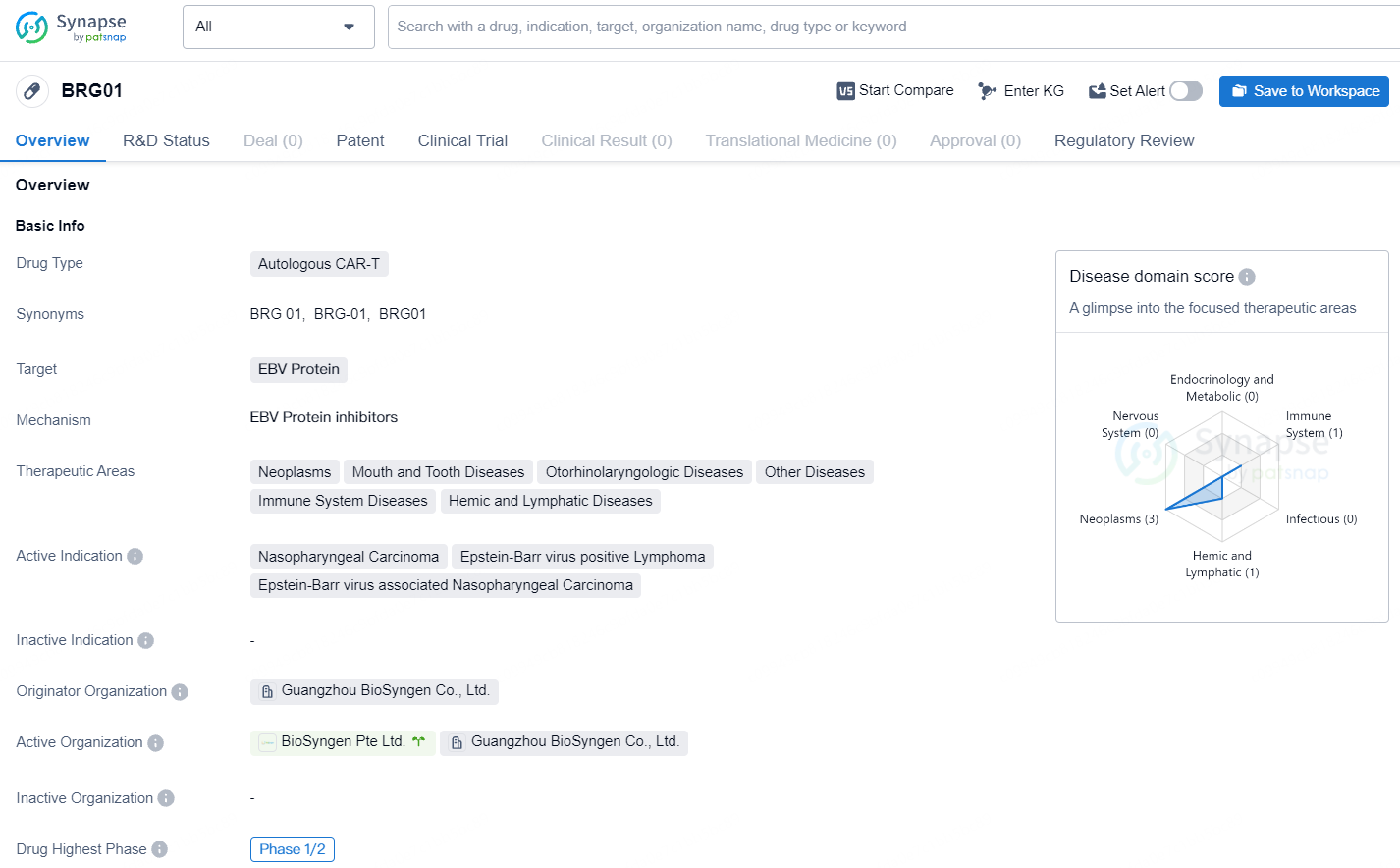
BRG01 is an autologous T cell immunotherapy engineered to express chimeric receptors targeting the Epstein-Barr virus antigen on T cell surfaces. This new generation CAR-T cell therapy is specifically designed to combat EBV. It received Phase I clinical trial approvals from the CDE in China in December 2022 and the FDA in the U.S. in February 2023. Furthermore, the FDA granted BRG01 orphan drug designation and fast track designation in June and July 2023, respectively.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
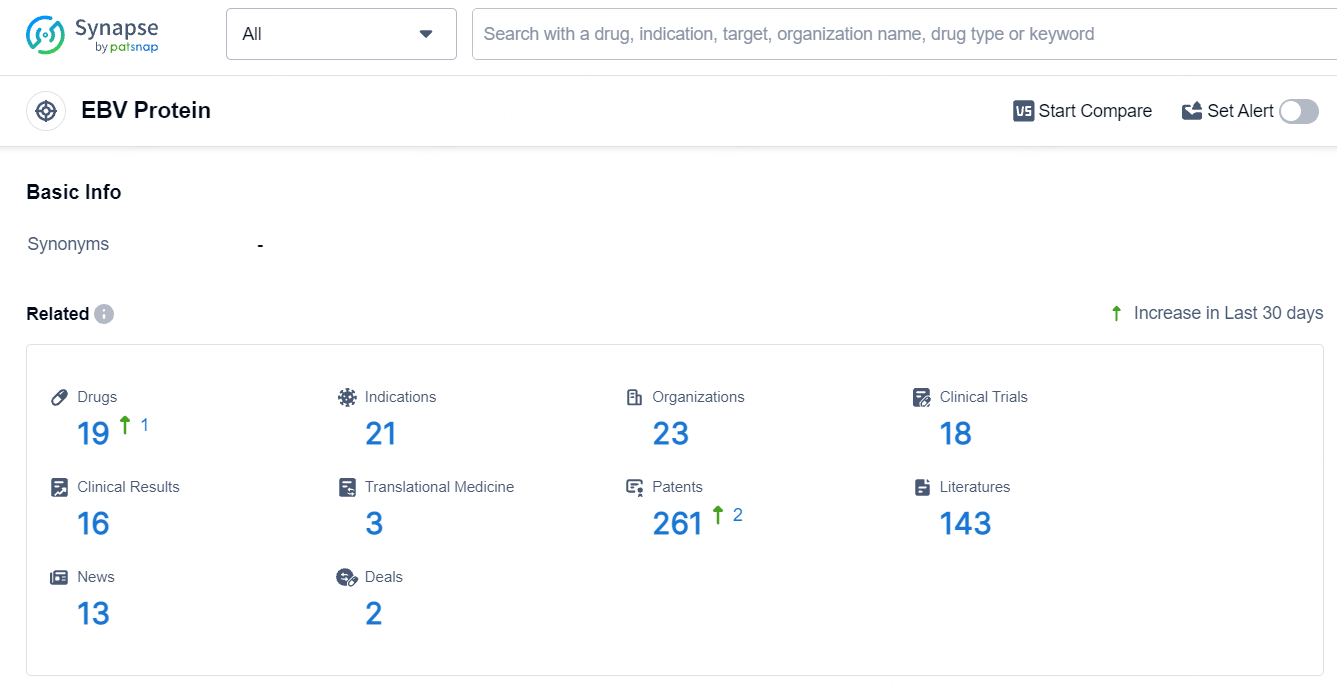
According to the data provided by the Synapse Database, As of July 22, 2024, there are 19 investigational drugs for the EBV target, including 21 indications, 23 R&D institutions involved, with related clinical trials reaching 18, and as many as 261 patents.
BRG01 represents a novel autologous CAR-T therapy with a specific focus on targeting EBV Protein for the treatment of nasopharyngeal carcinoma, Epstein-Barr virus positive lymphoma, and related conditions. Its current development status, regulatory designations, and therapeutic potential position it as a promising candidate in the field of biomedicine, with implications for the future treatment landscape of EBV-associated diseases.