Camurus Announces Positive Phase 3 Results for ACROINNOVA 2 Trial of Octreotide SC Depot (CAM2029) in Acromegaly Patients
Camurus revealed favorable final topline outcomes from the 52-week Phase 3 open-label ACROINNOVA 2 study, which assessed the safety and effectiveness of their once-monthly octreotide subcutaneous depot (CAM2029).
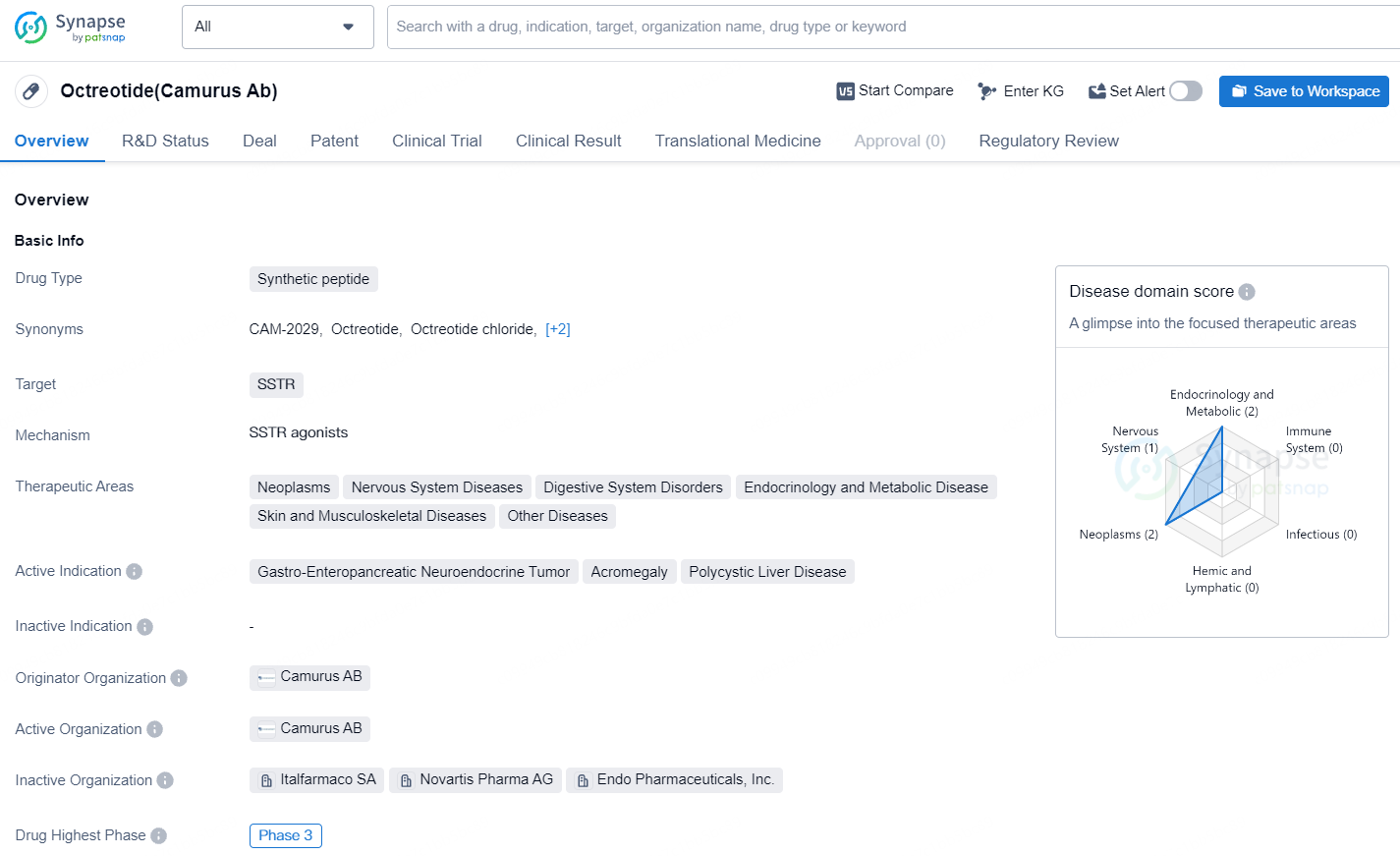
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Fredrik Tiberg, President, CEO, and CSO of Camurus, states: "The findings from ACROINNOVA 2 underscore the prolonged safety and effectiveness of octreotide SC depot for acromegaly patients, including those whose disease remains uncontrollable with standard therapies. This data bolsters the evidence supporting CAM2029 octreotide SC depot as a potential new treatment for acromegaly, pending approval. Regulatory assessments are in progress in both the US and EU, with the first potential approval from the US FDA anticipated around 21 October 2024, the PDUFA action date."
The main goal was to evaluate safety over a 52-week treatment period. Octreotide SC depot was well tolerated, displaying a long-term safety profile similar to that of standard-of-care treatments with first generation somatostatin receptor ligands, such as extended-release octreotide and lanreotide, with no novel safety concerns.
The most frequent side effects were mild to moderate reactions at the injection site and gastrointestinal issues. No severe adverse events linked to octreotide SC depot were reported. One patient experienced a treatment-related serious adverse event of cholelithiasis, which resolved, allowing the patient to continue the trial. Two patients stopped treatment due to adverse events: one due to mild depression and the other due to mild injection site hemorrhage.
Acromegaly is a rare, gradually progressing condition typically driven by a pituitary tumor that overproduces growth hormone, leading to heightened insulin growth factor-1 levels. This condition causes abnormal bone and tissue growth, enlarged extremities and facial features, and internal organ enlargement, accompanied by symptoms like fatigue, joint pain, headaches, visual disturbances, excessive sweating, and paresthesia.
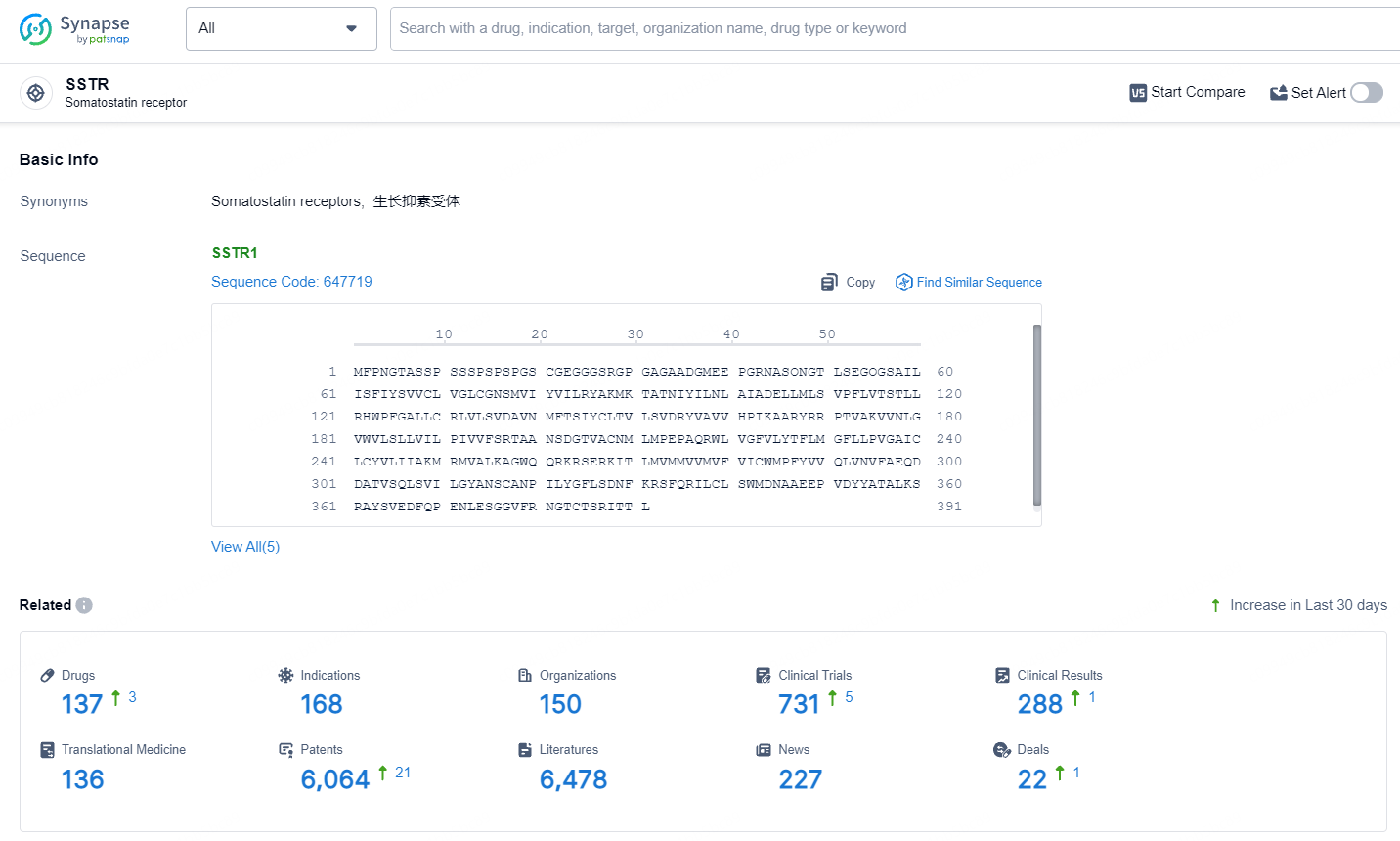
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 17, 2024, there are 137 investigational drugs for the SSTR target, including 168 indications, 150 R&D institutions involved, with related clinical trials reaching 731, and as many as 6064 patents.
CAM2029 is a synthetic peptide drug targeting SSTR with multiple therapeutic applications in various disease areas. It is currently in Phase 3 of development globally and holds promise for potentially treating rare conditions such as Gastro-Enteropancreatic Neuroendocrine Tumor, Acromegaly, and Polycystic Liver Disease. Its orphan drug status further underscores its importance in addressing unmet medical needs in these specific disease areas.