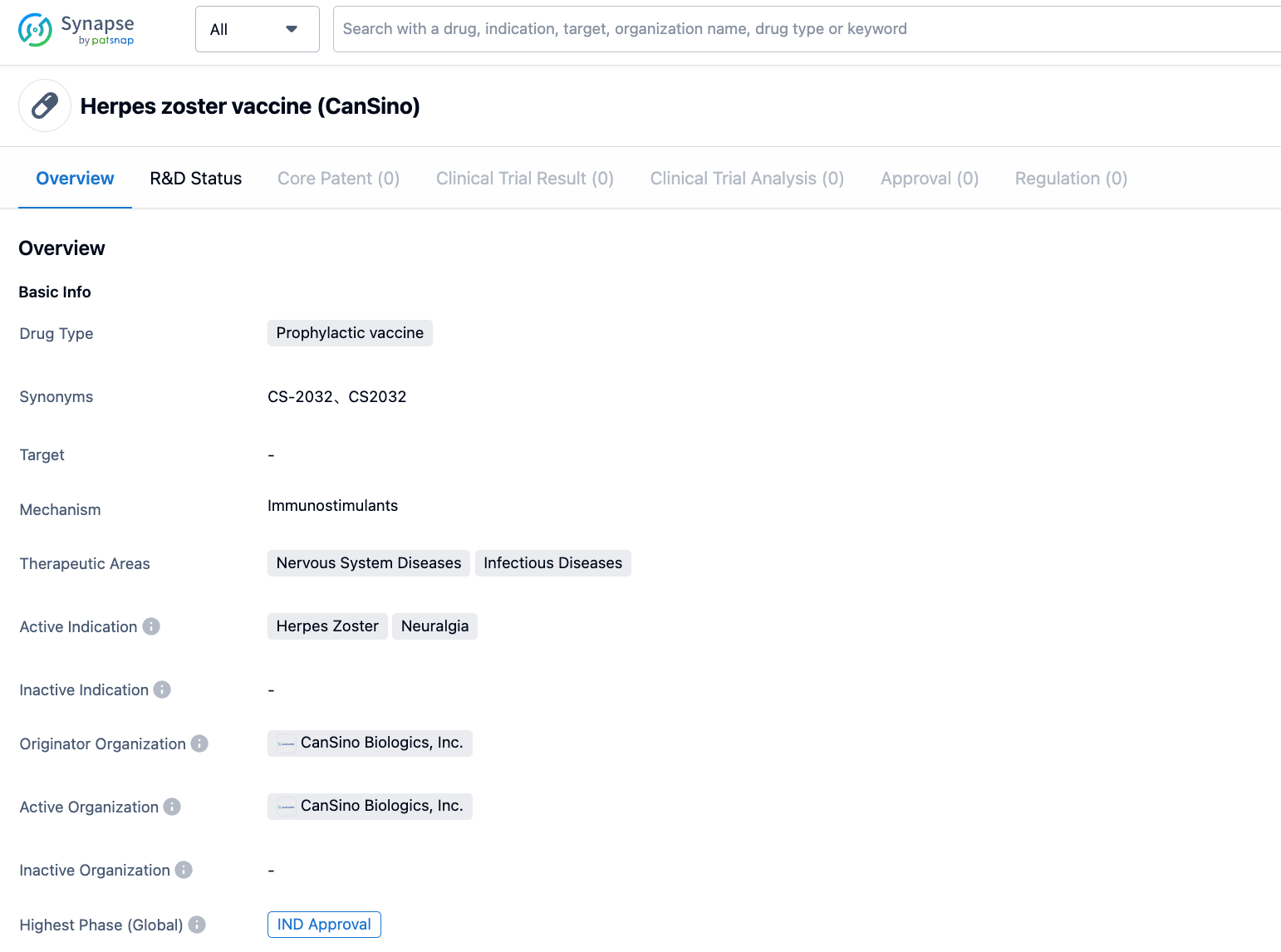
CanSino's Herpes Zoster vaccine CS-2032 has been approved for clinical use in Canada
On July 23, CanSino Biologics announced that its recombinant shingles vaccine CS-2032, developed in collaboration with Vaccitech, has received clinical trial approval from Health Canada. The vaccine is intended for adults over 50, and is designed to prevent skin shingles and neuralgia caused by the varicella-zoster virus. Clinical trials will be conducted to evaluate the vaccine's delivery both through intramuscular injection and inhalation methods.
The CS-2032 shingles vaccine utilizes the chimpanzee adenovirus vector technology route, which stimulates both cellular and humoral immunity. Among the shingles vaccines on the market globally, two primary technology routes are used: Zoster Vaccine Live (ZVL) and the Recombinant Zoster Vaccine (RZV). CS-2032, like GSK's Shingrix, is a recombinant vaccine, but CS-2032 uses the chimpanzee adenovirus as a vector, stimulating both cellular and humoral immunity.
Pre-clinical research data shows that there is no significant difference in humoral immunity between CS-2032 and the shingles vaccine, whereas the level of systemic cellular immunity is significantly higher than Shingrix. This suggests that CS-2032 demonstrates strong protective efficacy.
Shingles is an infectious skin disease caused by the reactivation of the varicella-zoster virus (the virus that causes chickenpox), most commonly occurring in individuals over the age of 50. Prior to the outbreak of the rash, there are often prodromal symptoms such as fatigue, low fever, and loss of appetite. When the rash develops, the affected skin first shows erythema and later erupts into clustered papules that subsequently transform into vesicles. The skin lesions usually distribute along a certain peripheral nerve region, generally occurring on one side of the body and not crossing the midline. In addition to the cutaneous manifestations, patients often suffer from varying degrees of neuropathic pain.
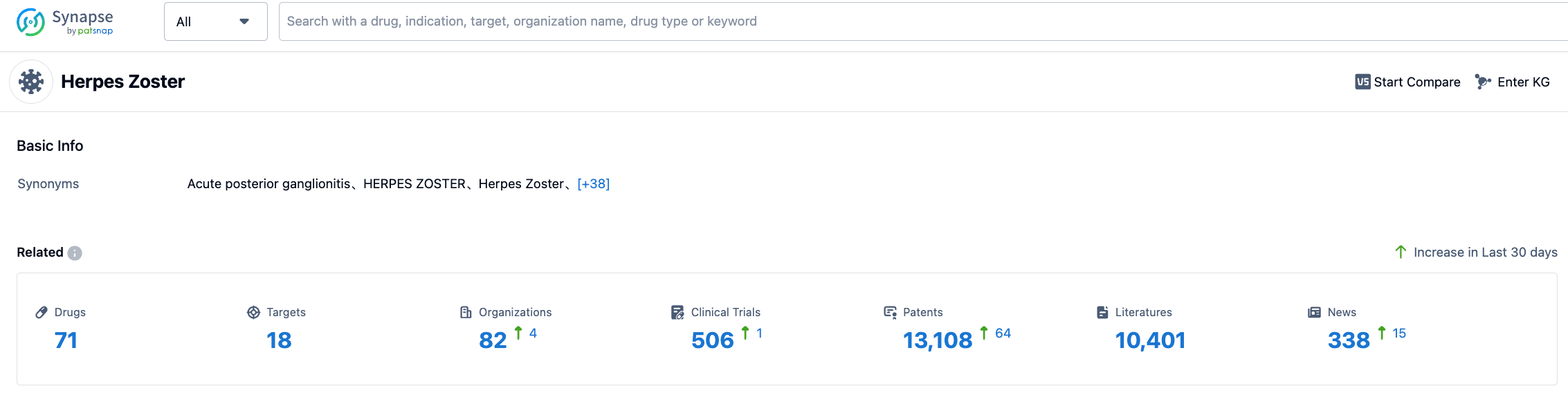
According to the information disclosed by Synapse (click on the card below to reach the Shingles indication, after registration and login, you can obtain detailed information about drugs under research, targets, research institutions, clinical trials, etc. under this indication for free), as of July 25, 2023, there are 71 drugs under research for the Shingles indication, involving 18 targets, 82 research institutions, 506 related clinical trials, and 13,112 patents. Shingles has a huge market, and we look forward to the future performance of CS-2032.