Carisma Reveals New Results from Initial Phase 1 Clinical Trial of CT-0508 at 8th Annual CAR-TCR Summit
Carisma Therapeutics Inc. is a biopharmaceutical enterprise in the clinical trial phase focused on the research and creation of pioneering immunotherapies. The firm's primary candidate product, CT-0508, is set to be displayed at this year's 8th annual CAR-TCR conference. This particular project involves a clinical investigation of human growth factor receptor 2 ("HER2") ending macrophages with chimeric receptors ("CAR M"), a potential treatment for progressive or widespread cancers marked by excessive HER2 expression.
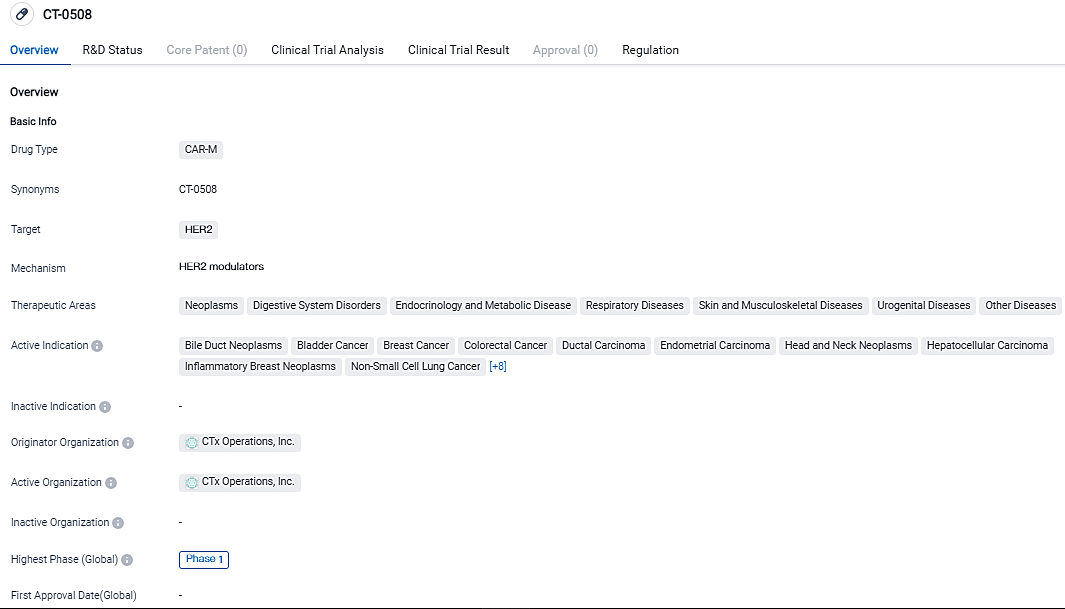
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The demonstration includes information from group 1 (n=9) and group 2 (n=5). Patients from both cohorts were administered the same total dosage (up to 5x109CT-0508) via either a segmented, multi-day infusion protocol or a one-day bulk infusion. The information comes from the ongoing clinical trial led by Kim A. Reiss, M.D., the main researcher in the Phase 1 clinical trial and an Assistant Professor of Hematology-Oncology at the Perelman School of Medicine at the University of Pennsylvania.
In addition, Michael Klichinsky, PharmD, Ph.D., Co-Founder and Lead Scientific Officer at Carisma, will give a demonstration providing evidence that, in both cohorts, CT-0508 was successfully produced for patients and was tolerated well after being infused with no recorded dose-limiting toxicity issues so far.
"Observing the steady and hopeful findings in relation to the safety, practicality, and functionality of the pioneering CAR-M investigational therapy in the CT-0508 trial is encouraging," remarked Dr. Klichinsky. "We anticipate the forthcoming outcomes of the CT-0508's combined sub-study with pembrolizumab and the persistent progression of CAR-M alongside CAR-Monocyte therapies."
Carisma previously publicized results from the first group indicating that CT-0508 altered and stimulated the tumor microenvironment ("TME") and initiated anti-tumor T cell immunity. Integrative analyses of both groups one and two reveal correlating various biomarkers, such as TME activity metrics, T cell activation, and HER2 status, with the most favorable response of stable disease. This provides additional proof of CT-0508's mode of operation.
The Phase 1 study's translational evaluations further exemplify an escalation in fatigued CD8 T cells while being treated, substantiating the ongoing combination sub-study with Merck's anti-PD1 therapy, KEYTRUDA®(pembrolizumab).
The most recent disclosure of data came after the first participant was given a dose in the continued sub-study of CT-0508 in conjunction with pembrolizumab for treating HER2 overexpressing cancers as part of the Phase 1 clinical trial.
Today, the business is submitting a Current Report on Form 8-K to the U.S. Securities and Exchange Commission, revealing new data from its Phase 1 clinical trial of CT-0508. This will include a segment of the presentation being showcased at the 8th Annual CAR-TCR Summit.
Carisma has obtained the rights to use specific intellectual property owned by the University of Pennsylvania from the said institution, with the Perelman School of Medicine at Penn receiving financial support for research and clinical trials from this company. There's a possibility that Penn could gain further financial advantage from technologies that have been licensed or offered as options to Carisma. Moreover, Penn is classified as a co-establisher of the company and has a stake in Carisma.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
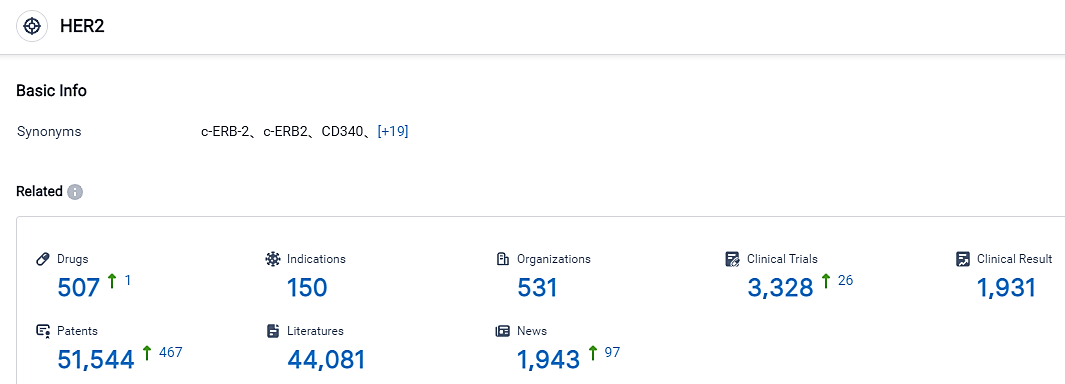
According to the data provided by the Synapse Database, As of September 3, 2023, there are 507 investigational drugs for the HER2 target, including 150 applicable indications, 531 R&D institutions involved, with related clinical trials reaching 3328, and as many as 51544 patents. CT-0508 is a chimeric antigen receptor macrophage targeting human epidermal growth factor receptor 2. It is currently undergoing an essential Phase 1 clinical trial across multiple centers, focusing on individuals wrestling with recurrent/metastatic solid tumors that overexpress HER2.
The trial is specifically designed for those cancer patients who either lack approved HER2-centered therapies or do not show any response to treatment. Carisma enlists trial participants with tumors of various anatomical origins, all united by the strategic overexpression of HER2 receptors on the cell membrane. This is the critical target for our novel CAR-M. This Phase 1 assessment signifies the inaugural study of bioengineered macrophages in human subjects.