CASI Pharmaceuticals Receives FDA Approval for New ITP Drug CID-103
CASI Pharmaceuticals, Inc., a company focused on the development and commercialization of novel therapeutic and pharmaceutical solutions, has made the following announcement: On April 12, 2024, CASI filed an IND application with the FDA for CID-103 to initiate a phase 1/2 clinical trial in adults suffering from chronic Immune Thrombocytopenia. Subsequently, on May 13, 2024, CASI received a notification from the FDA indicating that the study is allowed to proceed.
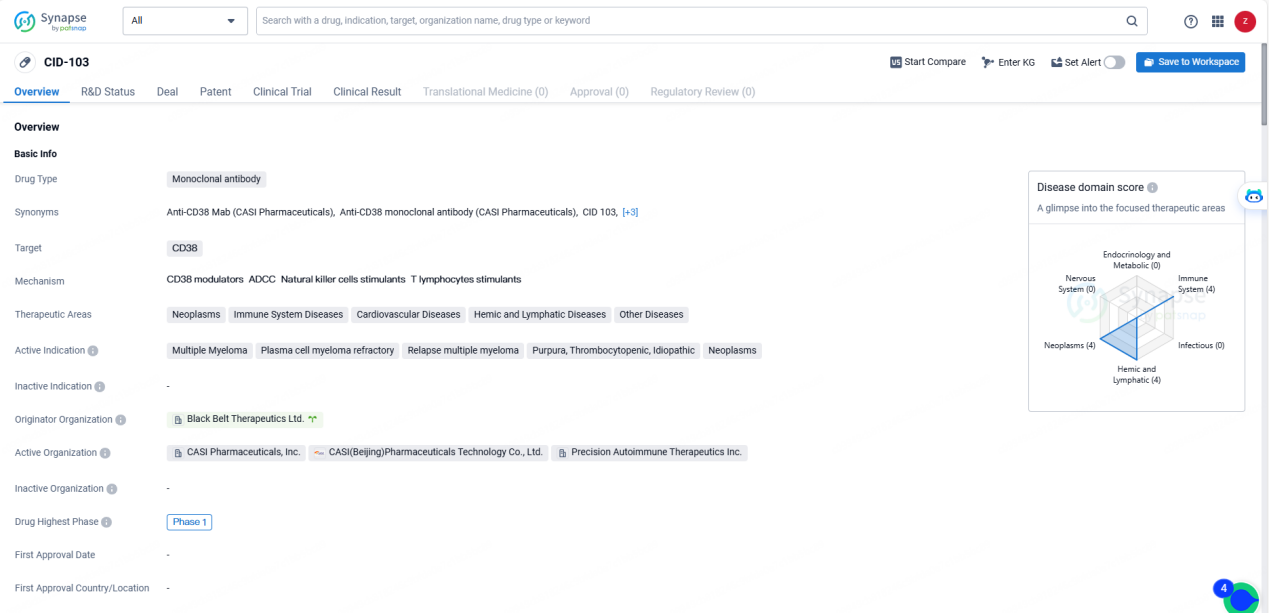
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
CID-103 is a fully human IgG1 anti-CD38 monoclonal antibody that targets a distinct epitope, exhibiting promising preclinical efficacy and safety relative to other anti-CD38 monoclonal antibodies.
Dr. Wei-Wu He, CEO of CASI, stated, "ITP is a critical autoimmune disorder of the blood marked by the destruction of platelets through autoantibodies and reduced platelet production, which results in thrombocytopenia and a higher risk of severe bleeding episodes. We are thrilled to move this initiative into clinical development because CID-103 holds the promise of becoming a novel therapeutic option that can potentially reduce the disease burden in this patient population."
CASI Pharmaceuticals, Inc. is a biopharmaceutical firm dedicated to the development and commercialization of cutting-edge therapeutics and pharmaceutical products in China, the United States, and globally. The company concentrates on acquiring, developing, and marketing products that enhance its hematology-oncology therapeutic portfolio as well as other areas with significant medical needs.
The company plans to execute its strategy to become a market leader by launching medications in the Greater China region, utilizing its regulatory and commercial capabilities based in China alongside its global drug development expertise. The company operates in China through its fully owned subsidiary, CASI Pharmaceuticals Co., Ltd., situated in Beijing, China. Additional information on CASI can be found at
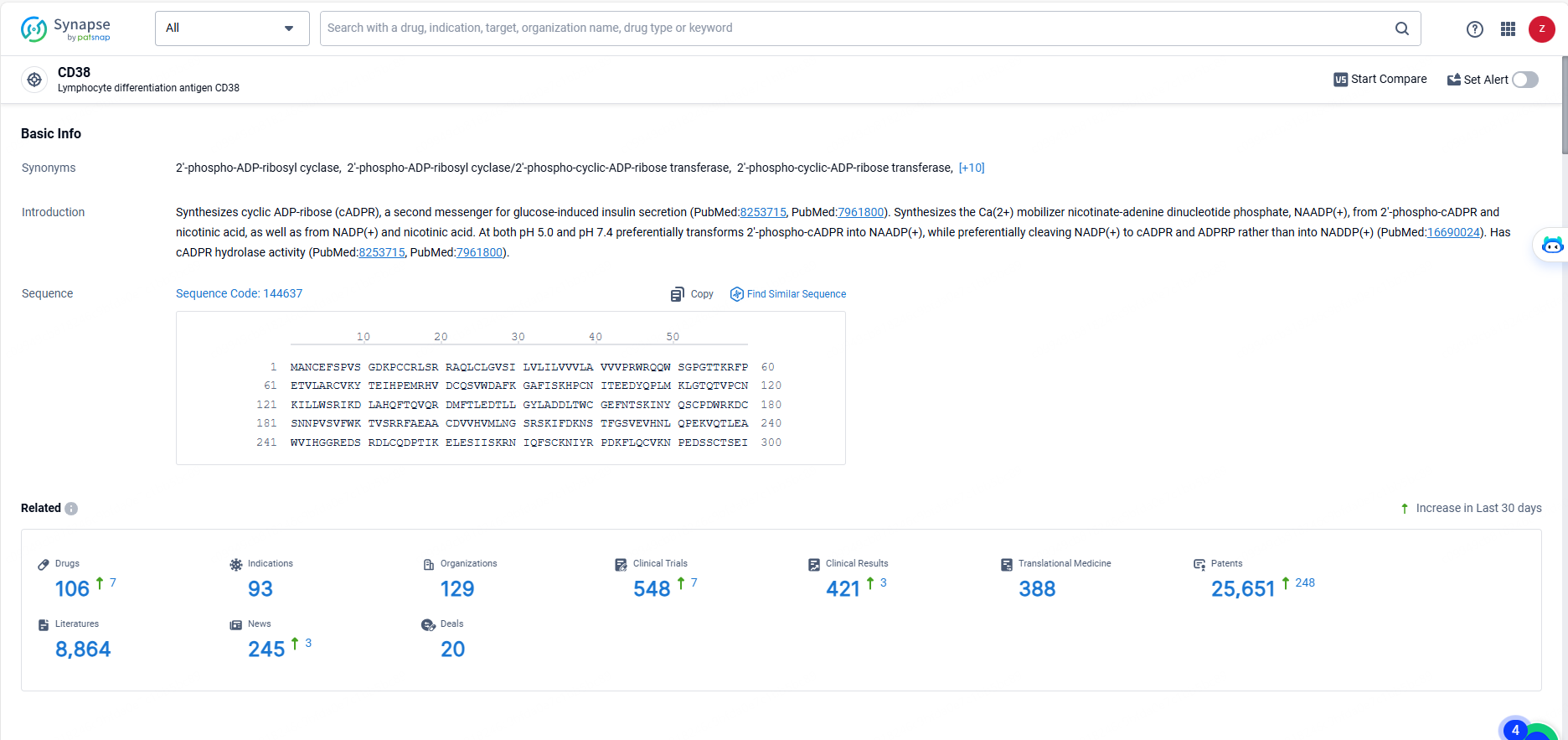
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 106 investigational drugs for the CD38 target, including 93 indications, 129 R&D institutions involved, with related clinical trials reaching 548, and as many as 25651 patents.
CID-103 is a monoclonal antibody drug targeting CD38, with potential applications in the treatment of various diseases, particularly in the field of oncology and immune system disorders. The drug has progressed to Phase 1 globally and has obtained an IND Application status in China, indicating its potential as a promising candidate for further clinical development and potential commercialization.