CD38 inhibitors——New Hope for Multiple Myeloma Patients with Medications
Multiple Myeloma (MM) is a malignant clonal plasma cell disease, accounting for about 10% of malignant tumors of the hematopoietic system. The prognosis of MM varies greatly, it is currently incurable, and most patients still face the problem of recurrence after treatment. With the emergence of new therapies such as immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and monoclonal antibodies (mAbs), the efficacy of MM has been significantly improved.
CD38 is a multifunctional enzyme found on the surface of various cells in the human body, including immune cells and certain tissues. It plays a crucial role in regulating calcium signaling and cell adhesion, as well as in the metabolism of NAD+ and cADPR. CD38 is involved in immune responses, cell proliferation, and differentiation.
The expression levels of CD38 molecules are relatively low in normal lymphocytes, myeloid cells, and non-hematopoietic tissue cells. However, CD38 is highly expressed in MM cells, making CD38 molecules an ideal target for MM treatment. Compared to traditional chemotherapy, anti-CD38 monoclonal antibodies have higher specificity and fewer toxic side effects. Due to their unique mechanisms of action, relatively low toxicity, and single-agent activity, anti-CD38 antibodies show great flexibility in monotherapy or combination treatment plans.
CD38 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 87 CD38 drugs worldwide, from 103 organizations, covering 56 indications, and conducting 556 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.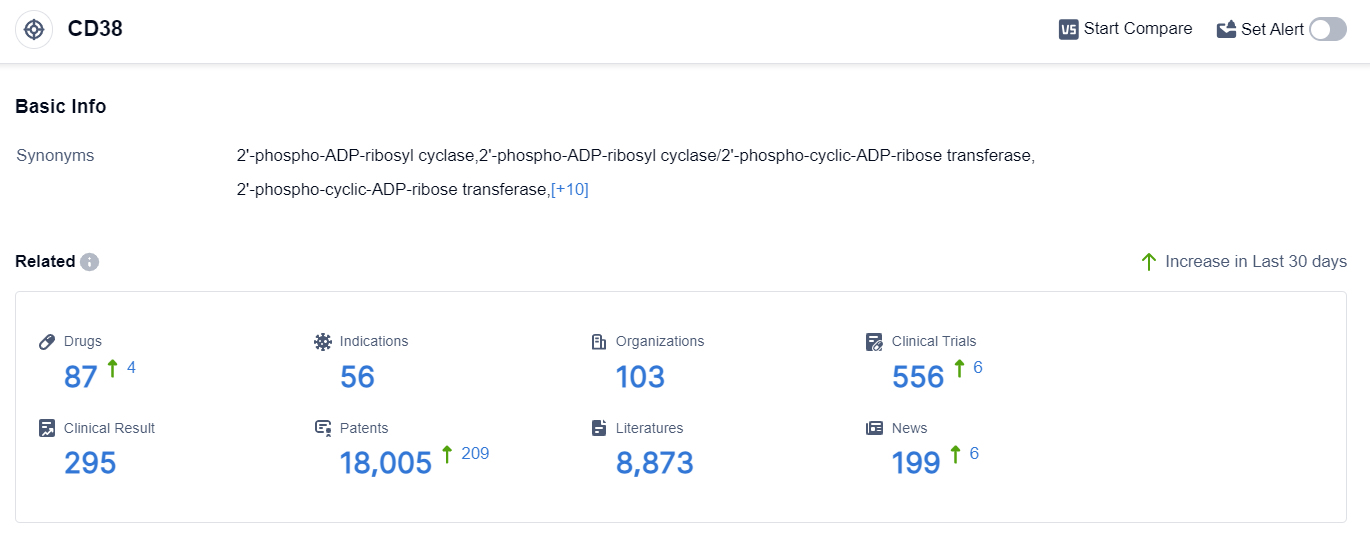
The current competitive landscape of target CD38 is characterized by the presence of multiple companies with drugs in different phases of development. Johnson & Johnson, Sanofi, Halozyme Therapeutics, Inc., I-MAB Biopharma Co., Ltd., and Takeda Pharmaceutical Co., Ltd. are the leading companies in terms of the highest stage of development.
The approved indications for drugs targeting CD38 include Multiple Myeloma, Light chain (AL) amyloidosis, and other hematologic neoplasms. Multiple Myeloma is the most common approved indication.
Monoclonal antibody, CAR-T, and Bispecific antibody are the drug types progressing most rapidly under the current target CD38. These drug types indicate intense competition around the innovative drug.
The United States, Japan, and China are the countries/locations with the highest development progress under the current target CD38. China, in particular, has shown significant progress with 2 drugs approved and several drugs in Preclinical phases.
Overall, the target CD38 presents a competitive landscape with multiple companies and drug types vying for development and approval. The future development of target CD38 is expected to continue with advancements in research and development, particularly in the areas of monoclonal antibodies, CAR-T, and bispecific antibodies.
Key Drug: Daratumumab
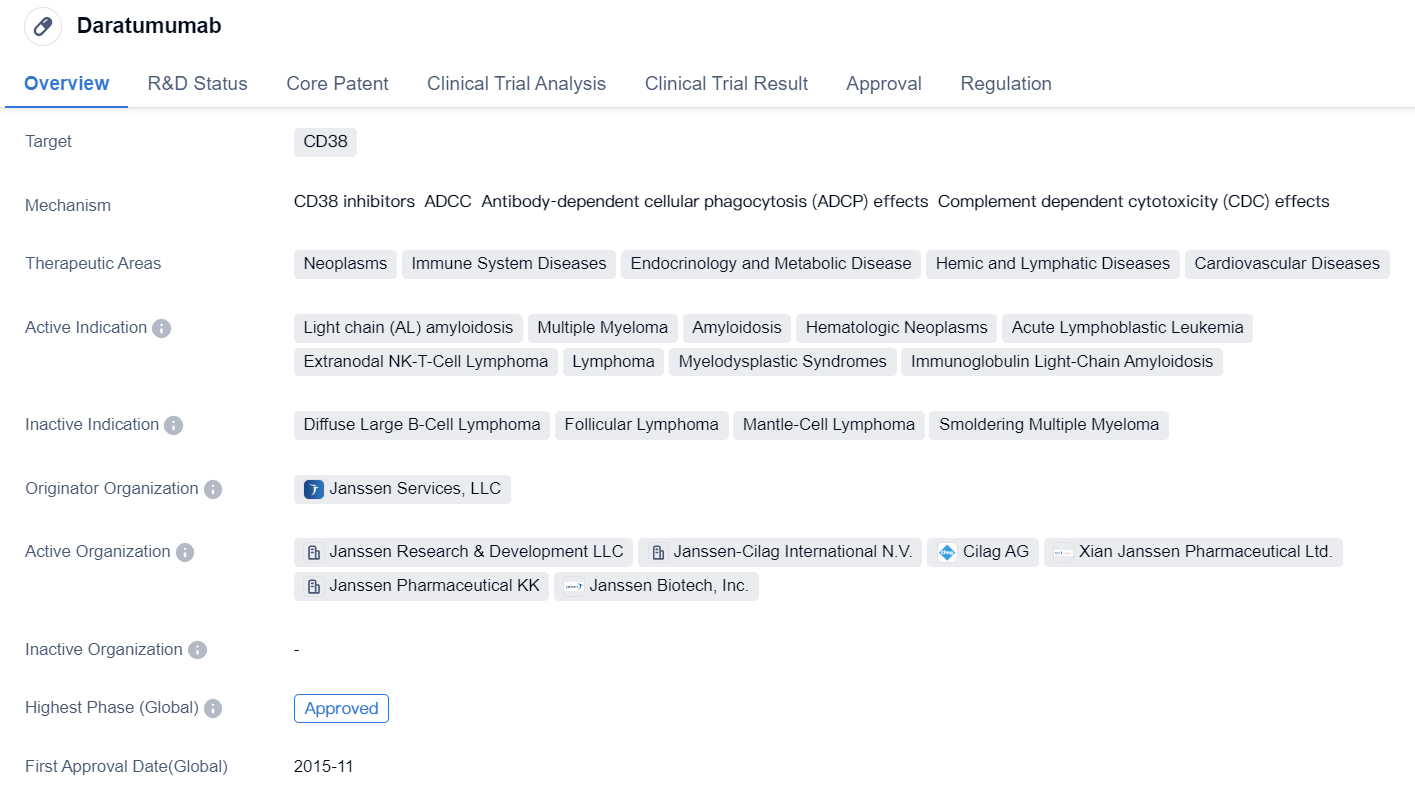
Daratumumab is a monoclonal antibody drug that targets CD38, a protein found on the surface of certain cells. It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, endocrinology and metabolic disease, hemic and lymphatic diseases, and cardiovascular diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating several indications, including light chain (AL) amyloidosis, multiple myeloma, amyloidosis, hematologic neoplasms, acute lymphoblastic leukemia, extranodal NK-T-cell lymphoma, lymphoma, myelodysplastic syndromes, and immunoglobulin light-chain amyloidosis.
Daratumumab was developed by Janssen Services, LLC, and it has received approval in both the global market and China. Its highest phase of development is approved, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
The drug received its first approval in the United States in November 2015. It has undergone various regulatory processes, including priority review, pediatric investigation plan, accelerated approval, fast track designation, orphan drug status, and breakthrough therapy designation. These regulatory designations highlight the potential significance and urgency of the drug in addressing unmet medical needs.
Daratumumab's approval and therapeutic areas suggest its potential in treating various diseases, particularly those related to the immune system and blood disorders. Its monoclonal antibody nature allows it to specifically target CD38, which may play a role in the pathogenesis of these diseases.
Overall, Daratumumab is a promising drug in the field of biomedicine, with a wide range of potential applications in the treatment of neoplasms, immune system diseases, endocrinology and metabolic disease, hemic and lymphatic diseases, and cardiovascular diseases. Its approval in multiple indications and regulatory designations further support its potential as a valuable therapeutic option.
Isatuximab-IRFC
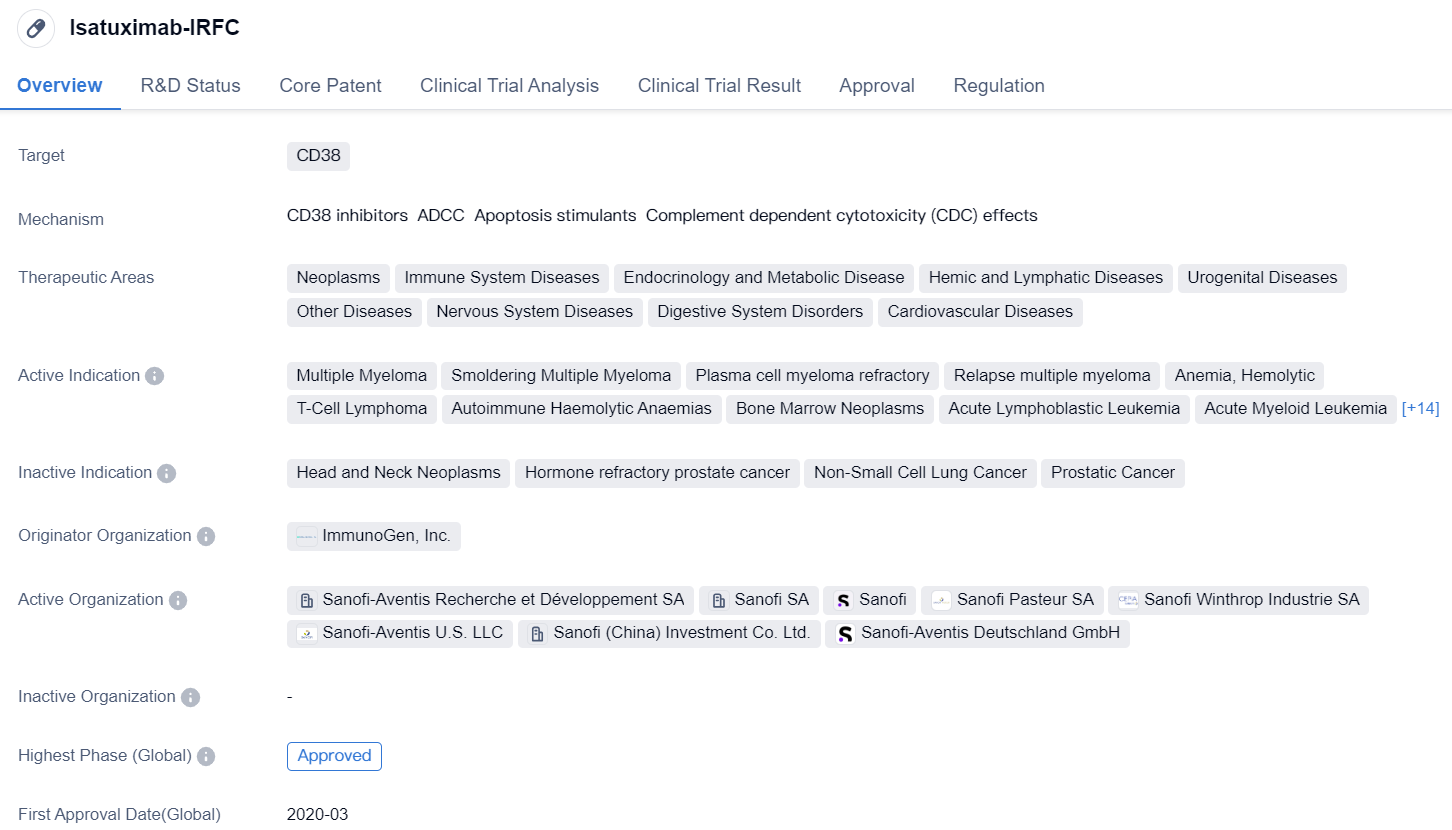
Isatuximab-IRFC is a monoclonal antibody drug that targets CD38, a protein found on the surface of certain cells. It has been developed by ImmunoGen, Inc. and has received approval for use in the treatment of multiple myeloma, a type of cancer that affects plasma cells in the bone marrow. The drug has also shown potential in the treatment of other diseases such as T-cell lymphoma, acute lymphoblastic leukemia, and colorectal cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Isatuximab-IRFC has been classified as an orphan drug, which means it is intended to treat rare diseases or conditions. This designation provides certain incentives to the manufacturer, such as market exclusivity and financial support for clinical trials.
The drug has gone through various phases of clinical trials, with the highest phase being approved globally. It received its first approval in the United States in March 2020. In China, it is currently in phase 3 of clinical development.
The therapeutic areas in which Isatuximab-IRFC is being explored include neoplasms (tumors), immune system diseases, endocrinology and metabolic diseases, hemic and lymphatic diseases, urogenital diseases, nervous system diseases, digestive system disorders, and cardiovascular diseases. This wide range of potential applications highlights the versatility of the drug and its potential to address various medical conditions.
Overall, Isatuximab-IRFC represents a significant advancement in the field of biomedicine, particularly in the treatment of multiple myeloma and other related diseases. Its approval and ongoing clinical development in different countries indicate its potential to provide effective therapeutic options for patients in need. As further research and clinical trials are conducted, it is expected that the drug's applications and benefits will continue to expand, potentially benefiting a larger population of patients with different medical conditions.