China's NMPA Accepts Innovent's NDA for IBI311 Antibody to Treat Thyroid Eye Disease
Innovent Biologics, Inc., an internationally recognized biopharmaceutical enterprise engaged in the development, production, and marketing of top-tier medications for cancer, metabolic disorders, autoimmune diseases, ophthalmology, and other significant health conditions, has declared that the New Drug Application (NDA) for IBI311, a recombinant antibody targeting the insulin-like growth factor 1 receptor (IGF-1R), has been accepted and assigned priority review status by the Center for Drug Evaluation of the China National Drug Administration for the treatment of Thyroid Eye Disease.
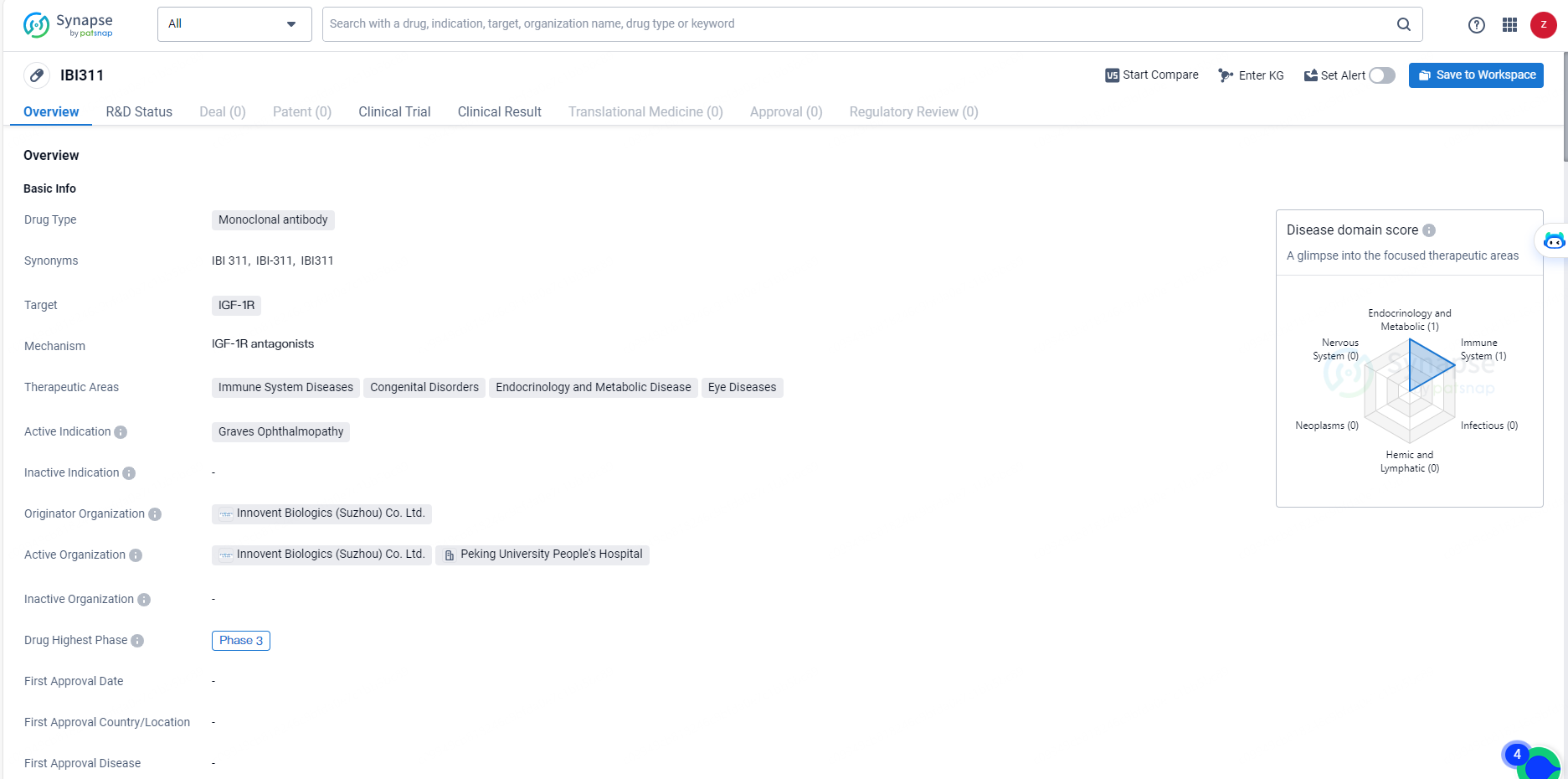
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
IBI311 stands as the first anti-IGF-1R antibody for which a New Drug Application (NDA) has been submitted in China. This biological drug, characterized by its unique mechanism of action, holds the potential to address a more than six-decade dearth of innovative treatments for TED (thyroid eye disease) in China, offering an effective, safe, and accessible therapeutic option for patients.
The NDA submission is underpinned by the successful outcomes of a Phase 3 registration clinical trial, RESTORE-1, conducted among TED patients in China. In February 2024, the trial met its primary endpoint. When compared to the placebo group, the IBI311 cohort exhibited marked improvements in proptosis, disease activity, and overall quality of life.
Professor Xianqun Fan of Shanghai Ninth People’s Hospital at Shanghai JiaoTong University School of Medicine, and the principal investigator of the RESTORE-1 trial, remarked, “I am immensely proud of the NDA submission, given the promising efficacy and safety profile IBI311 has demonstrated in this crucial study. I extend my gratitude to all investigators at the participating sites and look forward to IBI311 receiving approval. Such a development would signify a significant leap forward in managing TED, benefitting numerous Chinese patients.”
Dr. Lei Qian, Vice President of Clinical Development at Innovent, commented, “We are privileged to have our NDA for IBI311 accepted, thanks to the unwavering support and dedication of the trial participants, investigators, and regulatory authorities. IBI311 has shown promising safety and comprehensive efficacy in the RESTORE-1 study, which includes a reduction in proptosis, better control of disease activity, and enhanced quality of life. We are committed to ongoing communication with regulatory authorities throughout the NDA review process and aim to introduce this effective, safe, and accessible treatment option for Chinese TED patients at the earliest opportunity.”
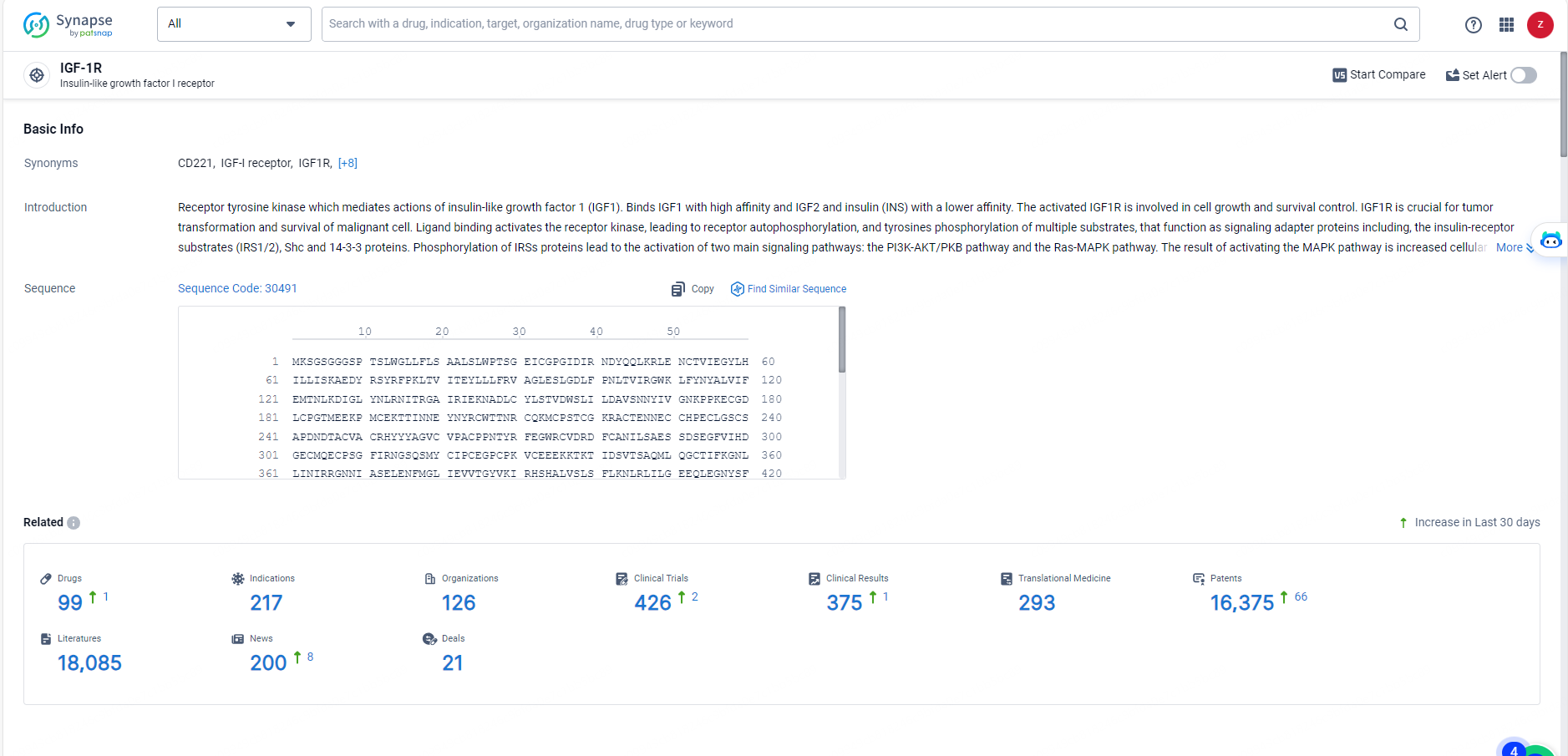
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 27, 2024, there are 99 investigational drugs for the IGF-1R target, including 217 indications, 126 R&D institutions involved, with related clinical trials reaching 426, and as many as 16375 patents.
IBI311 represents a promising development in the field of biomedicine, particularly for the treatment of Graves Ophthalmopathy and other related conditions. The drug's advancement to Phase 3 trials suggests that it has shown potential in early studies, and further research will be necessary to fully evaluate its safety and efficacy. As the drug progresses through clinical development, it may offer new treatment options for patients with immune system diseases, congenital disorders, endocrinology and metabolic diseases, and eye diseases.